2012-11-05
advertisement

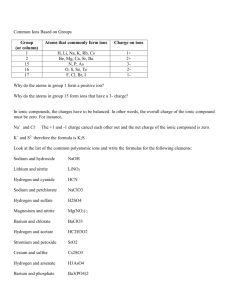
11/5/2012 Standards: 2 Objectives: a. Complete Zum - Formation of Ionic Cmpds (computer exercise) b. Be able to combine + and - ions in their proper ratios DO NOW: 1. Read and be able to report on Zum – Formation of Ionic Cmpds (today's lab) HOMEWORK: 1. Watch this video from YouTube. Write 1) Lattice Energy on first line. Go down 10 lines and write 2) Ionization Energy. Take 10 lines of notes on Lattice Energy and 2 lines on Ionization Energy. (first 4 min 30 sec) covers Lattice Energy. (7 min 15 sec – 9 min 15 sec) covers Ionization Energy (3pts) Std: 2 2. SN Ch 8.2 p. 97-98 (2 pts) Std: 2 ========================================================== NO STAMPING TODAY B LACK TRAY: Models of Ions - Cut & Paste – (5 pts) Std 2 WAIT !! ChemThink due tomorrow night by midnight Name: ____________ Date:______ Per:___ Models of Ions - Cut & Paste Mg2+ PO43- Mg2+ Mg2+ Mg2+ PO43- PO43- SO42- K1+ K1+ K1+ SO42- 11/6/2012 Standards: 2 Objectives: a. Be able to describe how ions are formed. b. Be able to describe and write notation for ionic bond formation. DO NOW: 1. Write the electron configuration for each of the following ions: Na1+ Cl1- S2HOMEWORK: 1. SN Ch 8.3 & 8.4 p. 99 – 102 (3 pts) Std 2 2. Ch 8.2 Assess p. 220 12-15 (2 pts) Std 2 =========================================================== STAMPS: Journal 11/5 1. SN Ch 8.2 p. 97-98 (2 pts) Std 2 2. Stamp: Watch this video from YouTube. Write " 1) Lattice Energy" on the first line. Go down 10 lines and write " 2) Ionization Energy". Take 10 lines of notes on Lattice Energy and 2 lines on Ionization Energy. ANNOUNCEMENTS: 1. Memorize Ions - 1 Thurs - 6 ions (6 pts) nitrate ion, acetate ion, sulfate ion, carbonate ion, phosphate ion, ammonium ion 2. IF YOU MISSED YESTERDATY'S LAB, or couldn't finish - make-ups during lunch or befor school 3. Pics of Friday's rally (194 pics & vids) at Photobucket.com/GHSActivities (GHSActivities part is case sensitive) Free downloads of anything you want. 4. ChemThink due tonight at midnight. Example of noble gas electron configuration for an element and its ion: the element B [He] 2s2 2p1 the ion B3+ [He] or 1s2 Show Interactive Chalkboard vid of reaction: 2 Na + Cl2 ----> 2 NaCl Example of chemical reaction Lattice Energies are always given as negative numbers SIZE Smaller ion ===> more lattice energy Larger ion ===> less lattice energy INDIRECT CHARGE Larger (+ or -) ===> more lattice energy DIRECT Charge more important than size 1 Which of these ionic compuounds has the highest lattice energy? A B 2 Which of these ionic compuounds has the highest lattice energy? A B 11/7/2012 Standards: 2 (Chemical Bonding), 5 (naming cmpds) Objectives: a. Mem. Ions Quiz 1 tomorrow b. Be able to predict relative lattice energies of different molecules c. Run ChemThink.com for Ionic Cmpds to check answers (vid on HW page) DO NOW: 1. Write the individual ions for potassium sulfide & magnesium oxide 2. Write correct molecule formulas for each. (use criss cross method) 3. Which of these 2 compounds has the most LE (lattice energy) and why? HOMEWORK: 1. Ch 8 Assess p. 236 67-73 (5 pts) Std 2 ======================================================== STAMPS: Journal 11/6 1. Stamp: SN Ch 8.3 & 8.4 p. 99 – 102 (3 pts) Std 2 2. Ch 8.2 Assess p. 220 12-15 (2 pts) Note for teacher This worked really well today 11/17/11. I gave the quiz & allowed 6 min to take it. Then I had students pick + & - ions from columns 1,2,3,6, & 7 only. Common errors discussed as they showed up: if subscript is 1, we don't write any subscript if both charges are = but opposite - we have no subscript, because we only need 1 of ea for net zero if subscripts are odd & even, simply use criss cross to get subscripts symbols must be written in capital letter. If symbol is 2 letter, 2nd letter is lower case It was difficult to do 3 questions, not enough time. Questions answered with responders Eric P1 Prop 30 passed :) Prop 32 defeated :) Prop 34 defeated :) Prop 38 defeated :( What can I say, were stuck with another 4 yrs of Obama. Good luck USA! :( DO NOW - figuring charge value & largest lattice energy potassium sulfide ions magnesium oxide ions potassium sulfide formula magnesium oxide formula Winner? Why? potassium sulfide charge value magnesium oxide charge value p Practice Problems 1. K & S 2. Mg & O K 1+ S Mg 2+ O 2- 2- K2S MgO *the subscripts cancel each other out 3. Na & F Na 1+ 1- F *the subscripts cancel each other out NaF Rules for Making Ionic Compounds Use the oxidation # of element. For all representative elements, group # determines oxidation #. Group 1 = 1+ Group 2=2+, Gp 3=3+, Gp 6=2-, Gp 7=1- EXAMPLE: Write the correct formula with the following 2 elements: Ca & Cl Ca 2+ 1- Cl Since the charges are different you will need to use subscripts to indicate the ratio of ions in the formula Ca Ca 2+ 1 1- Cl 1- Cl 2+ Step 2: Remove + and - signs CaCl2 ---> Step 1: Cross the charges over Ca 1 Cl Step 3: Write the completed formula, no 1's & smallest ratios --- 2 Use course-2-assignment-ionic-formulas.notebook to intro how ions bond ====> ratios of ions Practice putting a metal (columns 1-3) with a nonmetal (columns 6 or 7) 3 If the follwing 2 atoms react with each other to form an ionic molecule, what with the formula be? 49In + Se -----> 34 Answer ? 4 If the follwing 2 atoms react with each other to form an ionic molecule, what with the formula be? 11Na + S -----> 16 Answer? 5 If the follwing 2 atoms react with each other to form an ionic molecule, what with the formula be? 39Sr + 8O -----> Answer? 11/8/2012 Standards: 2 Objectives: a. Be able to define "sea of electrons, substitutional and Interstitial alloys b. Demoes of Mg + O2 ---> and conductivity of salts in water. c. Be able to name some simple ionic compounds d. Be able to put + & - ions together in their proper ratios DO NOW: 1. Slap-it for the following ions: ammonium, nitrate, sulfate, photphate, acetate, carbonate HOMEWORK: 1. Ch 8.4 Sec Assess, p. 231 40-44 (2pts) Std: 2 2. Ch 8 Assess, p. 237 80-84 (3pts) Std: 2 ======================================================== STAMPS: Journal 11/7 1. Ch 8 Assess p. 236 67-73 (5 pts) Std 2 ammonium, nitrate, sulfate, phosphate, acetate, carbonate See link in Web Helps > Ch 5 > Songs (the first one) 11/8/12 15 lines? YES BUT only 5 questions If you use pencil - you cannot get an error in grading corrected If you use pen - you can get an error in grading corrected, if and only if you do not have a cross out When done turn over quiz turn over ans sheet P3 stopped at formula on top 11/9/2012 Standards: 2 (Chemical Bonds) Objectives: a. Be able to define "sea of electrons, substitutional and Interstitial alloys b. Be able to name some simple ionic compounds c. Be able to put + & - ions together in their proper ratios DO NOW: 1. Write these pairs of atoms as ions, and then create the correct formulas they will form: a. sodium & acetate for the molecules b. beryllium and carbonate HOMEWORK: 1. HANDOUT - Writing Chemical Formulas from Ions (5 pts) Std 2 2. Card Dec for: Chromate ion, dichromate ion, chlorite ion ======================================================== BASKET: Journal 11/8 1. Ch 8.4 Sec Assess, p. 231 40-44 (2pts) Std: 2 2. Ch 8 Assess, p. 237 80-84 (3pts) Std: 2 DO NOW Rules for Making Ionic Compounds Use the oxidation # of element. For MOST representative elements, group # determines oxidation #. EXAMPLE: Write the correct formula with the following 2 elements: Ca & Cl 2+ Ca 1- Cl Since the charges are different you will need to use subscripts to indicate the ratio of ions in the formula 1 Step 1: Cross the charges over TAKE ABSOLUTE VALUE Ca 1 CaCl2 Cl 2 Step 2: Write the completed formula, no 1's & smallest ratios Naming Ionic Molecules Metal or positive ions (always written first) use name of metal or name of positive ion Non-metal (negative) Ions of single atoms - name of atom but use ending -ide Polyatomic - use name of ion (see mem. ions) ctice Pra Na carbonate Ba sulfate Be acetate Ca chlorine Mg sulfur Al oxygen ANSWERS: Na2CO3 BaSO4 Al2O3 Be(C2H3O2)2 CaCl2 MgS Homework questions from last night Note blue haze represents “sea of electrons - text p. 228 Show molecules of different types and how they are packed (other laptop)