Molecular Shapes
advertisement
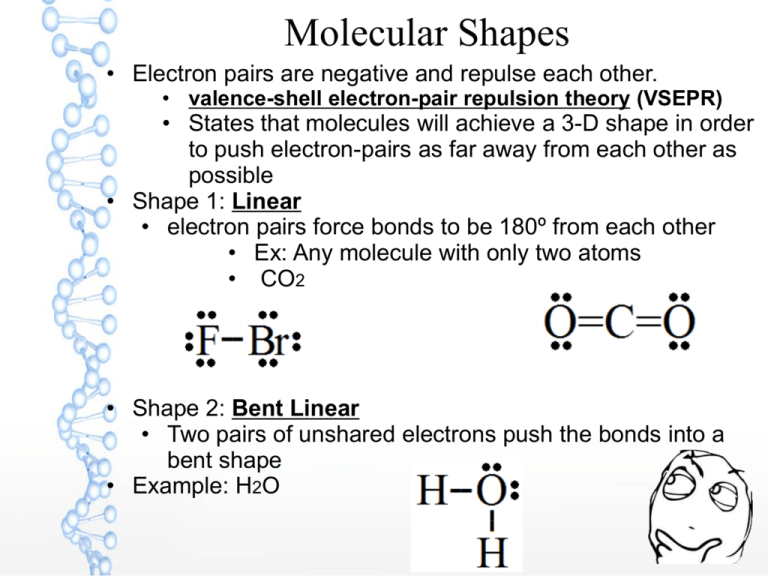
Molecular Shapes • Electron pairs are negative and repulse each other. • valence-shell electron-pair repulsion theory (VSEPR) • States that molecules will achieve a 3-D shape in order to push electron-pairs as far away from each other as possible • Shape 1: Linear • electron pairs force bonds to be 180º from each other • Ex: Any molecule with only two atoms • CO2 • Shape 2: Bent Linear • Two pairs of unshared electrons push the bonds into a bent shape • Example: H2O Molecular Shapes Shape 3: (Trigonal) Pyramidal – A single pair of unshared electrons push bonds away into a pyramidal shape Example: Ammonia, NH3 Shape 4: Tetrahedral – With a central atom that bonds 4 times(Carbon), the bonds are spaced out equally. Example: Methane, CH4 Molecular Shapes Shape 5: Trigonal Planar • A central atom bonded to three elements with no unshared pairs of electrons. • Shape is triangular and flat • Examples: BH3, CH2O Molecular Polarity · Polarity is the asymmetrical arrangement of charges in a molecule · Molecules can be polar or nonpolar based on their shape or their symmetry. Nonpolar molecules have symmetry. – no positive/negative end – charge is evenly distributed Examples: Diatomic molecules(BrINClHOF), CH4, CO2 Polar molecules do not have symmetry – Linear, trigonal planar and tetrahedral shapes can be polar if there is no symmetry – Bent linear and pyramidal shapes are always polar Intermolecular Forces of Attraction • Molecule- compounds that are formed by covalently bonded elements • Molecules tend to be • soft or brittle • Poor conductors of heat & electricity • low melting points • Many are gases or liquids at room temperature • Intermolecular Forces of Attraction • Determines the phase of molecules(solid, liquid or gas) • Dipole-dipole forces- polar molecules tend to “stick” to each other because of their positive-to-negative bonding. • Van der Waals Forces- non-polar molecules have a weak attraction to each other • Dipole-dipole forces are stronger than Van der Waals forces • Ionic bond > metallic bond > dipole-dipole > Van der Waals Physical Properties of Molecules “Like dissolves like” is a concept that explains why: • Polar solvents dissolve polar solutes • Nonpolar solvents dissolve nonpolar solutes • The positive and negative ends of polar molecules line up with each other, allowing polar solutes to dissolve. • Nonpolar solutes will dissolve because their intermolecular forces are weak, allowing them to dissolve. • Nonpolar and polar do not mix because the intermolecular forces of nonpolar solutes(aka Van Der Waals) cannot overcome the intermolecular forces of polar solutes (dipoledipole forces). Physical Properties of Molecules · Ionic solutes can dissolve in polar solvents because polar solvents will move their positive ends to the negative ion and their negative end to the positive ion. · This allows the ions to travel easily in a solution (and conduct electricity) Ternary ionic compounds have both ionic and covalent bonds. Polyatomic ions are made from covalent bonds, but can gain or lose electrons to form an ion Naming Binary Molecules Step 1: Determine if ionic or molecular. If it starts with a metal, it is ionic. See ionic rules for naming salts. Step 2: The more metallic(closer to francium) atom is written first Step 3: The least metallic (closer to flourine) atom is written second & ending is changed to “-ide.” Step 4: Add prefixes to indicate how many of each element is in the formula. Mono is only used for the second element. CO2 = carbon dioxide # atoms Prefix Examples: – SO2 – CCl4 – N2H4 sulfur dioxide carbon tetrachloride dinitrogen tetrahydride 1 Mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca