CP CHEMISTRY, Thermochemistry and Reaction Rates Review
advertisement

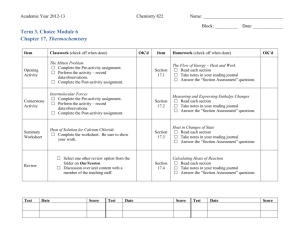
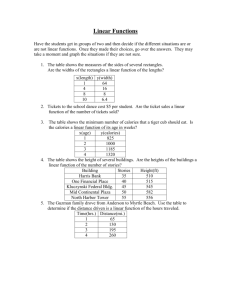
CP CHEMISTRY Name: ____________________________ Thermochemistry and Reaction Rates Review ___ period MUST KNOW ALL TERMS FOUND ON THERMOCHEMISTRY AND REACTION RATES VOCABULARY WORKSHEET!!! 1. What is energy? 2. What is heat? 3. What are the two types of energy? Describe them. 4. What is a calorie? 5. How is a food calorie different from a calorie? 6. What are the conversion factors for calories, Calories, and joules? 7. Perform the following conversions: a. 2180 calories = ? Calories CP CHEMISTRY, Thermochemistry and Reaction Rates Review, page 2 7. Perform the following conversions, cont’d: b. 82.5 J = ? calories c. 35 Calories = ? calories d. 450 Calories = ? kcal e. 675 calories = ? kJ 8. What is specific heat? 9. What is the equation for heat calculations? 10. If the temperature of a 250 g bar of aluminum increases from 15oC to 85oC, how much heat is absorbed by the aluminum? Aluminum has a specific heat of 0.897 J/g oC. CP CHEMISTRY, Thermochemistry and Reaction Rates Review, page 3 11. A nugget of pure gold absorbs 345 J of heat to raise its temperature from 22oC to 78oC. The specific heat of gold is 0.129 J/g oC. What is the mass of the gold nugget? 12. What is the specific heat of a metal if the temperature of a 12.5 g sample increases from 19.5 oC to 33.6 oC when it absorbs 37.7 J of heat? 13. Do all reactions occur at the same rate? Explain. 14. What is the equation for reaction rate? 15. What is the reaction rate of reaction in which the concentration of NaCl increases from 0.5 M to 4.2 M in 32 seconds? CP CHEMISTRY, Thermochemistry and Reaction Rates Review, page 4 16. Determine the reaction rate of a 1.6 M concentration of sulfur when it changes during a reaction to 0.3 M in 15 seconds. 17. What is the collision theory? 18. What three requirements MUST be met for a reaction to occur? 19. What forms if there is NOT enough activation energy in the activated complex? 20. What happens to the energy in an exothermic reaction? Draw a picture of the energy graph. 21. What happens to the energy in an endothermic reaction? Draw a picture of the energy graph. 22. What are the five factors that affect reaction rate? List them below and describe specifically how they affect reaction rate. a. CP CHEMISTRY, Thermochemistry and Reaction Rates Review, page 5 22. Five factors that affect reaction rate, cont’d. b. c. d. e. 23. What is an inhibitor and how does it affect reaction rate? CP CHEMISTRY, Thermochemistry and Reaction Rates Review, page 6 24. Reaction rate graph – plot the data, label the graph properly, and answer the question. Time (seconds) 0 5 10 20 25 30 a. Amount product (g) 0 12 21 32 43 51 How much product is formed at 15 seconds?