Acid Base Review squares
advertisement
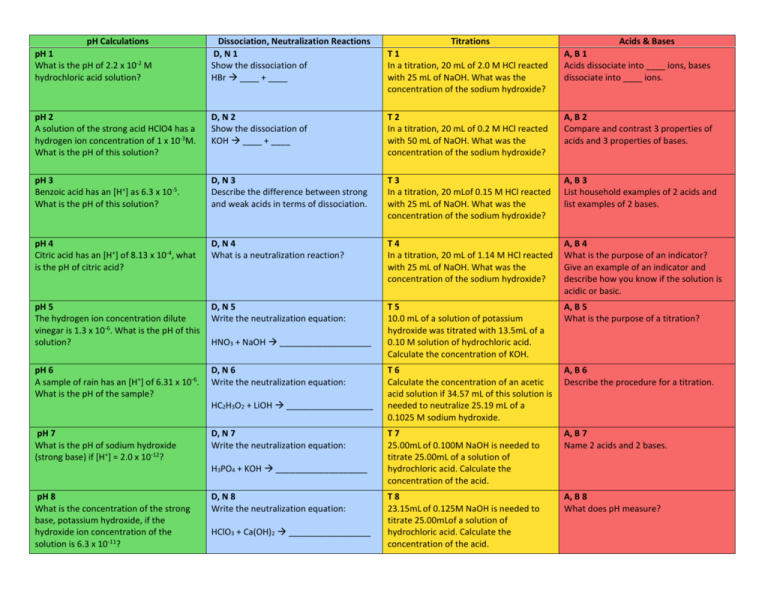
pH Calculations pH 1 What is the pH of 2.2 x 10-2 M hydrochloric acid solution? Dissociation, Neutralization Reactions D, N 1 Show the dissociation of HBr ____ + ____ Titrations T1 In a titration, 20 mL of 2.0 M HCl reacted with 25 mL of NaOH. What was the concentration of the sodium hydroxide? Acids & Bases A, B 1 Acids dissociate into ____ ions, bases dissociate into ____ ions. pH 2 A solution of the strong acid HClO4 has a hydrogen ion concentration of 1 x 10-3M. What is the pH of this solution? D, N 2 Show the dissociation of KOH ____ + ____ T2 In a titration, 20 mL of 0.2 M HCl reacted with 50 mL of NaOH. What was the concentration of the sodium hydroxide? A, B 2 Compare and contrast 3 properties of acids and 3 properties of bases. pH 3 Benzoic acid has an [H+] as 6.3 x 10-5. What is the pH of this solution? D, N 3 Describe the difference between strong and weak acids in terms of dissociation. T3 In a titration, 20 mLof 0.15 M HCl reacted with 25 mL of NaOH. What was the concentration of the sodium hydroxide? A, B 3 List household examples of 2 acids and list examples of 2 bases. pH 4 Citric acid has an [H+] of 8.13 x 10-4, what is the pH of citric acid? D, N 4 What is a neutralization reaction? T4 In a titration, 20 mL of 1.14 M HCl reacted with 25 mL of NaOH. What was the concentration of the sodium hydroxide? A, B 4 What is the purpose of an indicator? Give an example of an indicator and describe how you know if the solution is acidic or basic. pH 5 The hydrogen ion concentration dilute vinegar is 1.3 x 10-6. What is the pH of this solution? D, N 5 Write the neutralization equation: T5 10.0 mL of a solution of potassium hydroxide was titrated with 13.5mL of a 0.10 M solution of hydrochloric acid. Calculate the concentration of KOH. A, B 5 What is the purpose of a titration? pH 6 A sample of rain has an [H+] of 6.31 x 10-6. What is the pH of the sample? D, N 6 Write the neutralization equation: T6 Calculate the concentration of an acetic acid solution if 34.57 mL of this solution is needed to neutralize 25.19 mL of a 0.1025 M sodium hydroxide. A, B 6 Describe the procedure for a titration. T7 25.00mL of 0.100M NaOH is needed to titrate 25.00mL of a solution of hydrochloric acid. Calculate the concentration of the acid. A, B 7 Name 2 acids and 2 bases. T8 23.15mL of 0.125M NaOH is needed to titrate 25.00mLof a solution of hydrochloric acid. Calculate the concentration of the acid. A, B 8 What does pH measure? HNO3 + NaOH ___________________ HC2H3O2 + LiOH __________________ pH 7 What is the pH of sodium hydroxide (strong base) if [H+] = 2.0 x 10-12? D, N 7 Write the neutralization equation: H3PO4 + KOH ___________________ pH 8 What is the concentration of the strong base, potassium hydroxide, if the hydroxide ion concentration of the solution is 6.3 x 10-11? D, N 8 Write the neutralization equation: HClO3 + Ca(OH)2 _________________