Unit 4 Test Review
advertisement

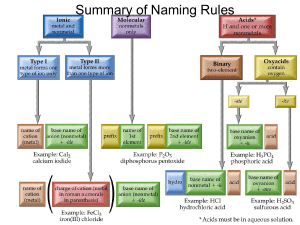
Unit 4 Review Sheet Name:__________________________________________________ _________1. Which formula represents an ionic compound? A. NaCl B. N2O C. P2O4 D. H2O _________2. A compound is created between chlorine and an unknown element X. If the formula of the compound is XCl, element X could be A. Sr B. Cs C. Al D. Ne _________3. In a sample of Ba(NO3)2 the ratio of barium ions to nitrate ions is A. 1:1 B. 1:2 C. 1:3 D. 1:6 _________ 4. Atom X is in Group 2 and atom Z is in group 15. A compound formed between these two elements would have the formula A. XZ B. X2Z5 C. X2Z3 D. X3Z2 C. Pb2O D. Pb2O3 _________5. What is the formula for lead(II) oxide? A. PbO B. PbO2 _________6. What is the charge on the metal in Mn3P4? A. 2+ B. 4+ C. 3+ D. 7+ _________7. What compound is formed when calcium and chlorine combine? A. CaCl B. CaCl2 C. Ca2Cl2 D. Ca2Cl _________8. In the formula XF2 the element represented by X can be classified as a(n) A. alkali metal B. alkaline earth metal C. halogen D. noble gas _________9. What is the chemical formula for zinc carbonate? A. ZnCO3 B. Zn(CO3)2 C. Zn2CO3 D. Zn3CO3 _________10. What is the correct name of the compound with the formula NH4NO2? A. ammonia nitrate C. ammonia nitrate B. ammonium nitrite D. ammonium nitrate _________11. Which is the formula of a binary compound? A. KOH B. NaClO3 C. Al2S3 D. Bi(NO3)3 Unit 4 Review Sheet Name:__________________________________________________ _________12. What is the correct name for the compound with the formula CrPO4? A. Chromium (II) phosphate C. Chromium (II) phosphide B. Chromium (III) phosphate D. Chromium (II) phosphide _________13. The correct name of the compound with the formula PbO2 is A. Lead (I) oxide C. Lead (III) oxide B. Lead (II) oxide D. Lead (IV) oxide _________14. The name of the compound KClO2 is potassium A. hypochlorite B. chlorite C. chlorate D. perchlorate _________15. What is the correct formula for carbon trinitride? A. C3N B.CN3 C. C3N3 D. CN _________16. What is the correct name for P4O10? A. quatraphosphorus decoxide C. tetraphosphide decoxide B. phosphorus oxide D. tetraphosphorus decoxide Short answer A. Name the following ionic compounds. 1. NaCl_________________________________ 3. AlPO4_______________________________ 2. Sr(NO3)2_____________________________ 4. Cs2O________________________________ B. Write the formula of the following ionic compounds. 1. Calcium sulfate_____________ 3. Nickel(III) oxide_____________ 2. Iron(III) chloride_____________ 4. Zinc thiosulfate_____________ C. Name the following ionic compounds using the stock system. 1. PdCl4________________________________ 3. Fe2O3________________________________ 2. V3(PO4)5______________________________ 4. Nb(NO2)3_____________________________ Unit 4 Review Sheet Name:__________________________________________________ D. Name the following covalent compounds. 1. CO2________________________________ 3. P3O9_________________________________ 2. SBr6________________________________ 4. N2O________________________________ E. Write the formula of the following covalent compounds. 1. dihydrogen monoxide_____________ 3. dinitrogen tetroxide_____________ 2. boron trisulfide_____________ 4. silicon dichloride_____________ F. Name the following compounds (Hint categorize them first as ionic(i), stock system(t) or covalent (c). 1. MgCl2________________________________ 4. CoN__________________________________ 2. SO4__________________________________ 5.AgC2H3O2______________________________ 3.CuClO4________________________________ 6. CF4 _________________________________ G. Write the formula for the following compounds (Hint categorize them first as ionic(i) or covalent (c). 1. rubidium sulfide_____________ 4. carbon dioxide_____________ 2. diphosphorus triodide_____________ 5. sodium sulfate _____________ 3. tin(IV) nitrate_____________ 6. manganese(III) nitride_____________