Organic Chem Textbook Chapter
advertisement

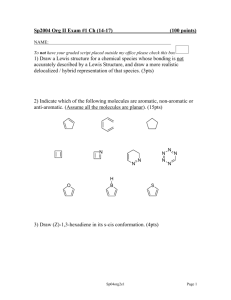
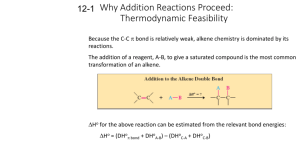
Organic chemistry is the chemistry of carbon compounds. Carbon is special because of its bonding. Carbon can make 4 bonds. It can bond to a variety of metals and non-metals. Carbon is capable of catenation, that is, it can form long chains with other carbon atoms with covalent bonds. Organic compounds are important in biology and industry. Let's look at how organic reactions are written: Reagent(s) Catalyst Product Reactant Solvent Conditions The main reaction of alkanes we need to know about is radical halogenation. It is called radical because the intermediates involved in the reaction are radicals, chemical species with unpaired electrons. CH4 + Cl2 = CCl4, CHCl3, CH2Cl2, and CH3Cl We have little control over the reaction so all four products are made. The reaction works with bromine as well. Fluorine reacts very violently and iodine doesn't really react as the carbon iodine bond is weak. HBr Let's look at the mechanism of the reaction: Br- Br- The important thing to note here is that the first step of the reaction involves a carbocation. The carbocation will be at the alkene carbon that does not bond to the hydrogen. The more substituted carbocation is more stable. Substitution refers to the number of carbons attached to the carbon that contains the carbocation: Primary, secondary and tertiary carbocations are seen above. Br2 H2O Let's look at the mechanism of the reaction: H3O+ Let's look at the mechanism of the reaction: Br- Br- H+ H2O Alkynes react similarly to alkenes. There are some differences though. 1 equiv Br2 2 equiv Br2 H2O Of interest with the above are the regioselctivity of the first reaction and the production of the carbonyl (C double bonded to O) group in the third reaction. In the first process both bromines add to the same carbon because of the stability of the intermediate: Br- Br- Br- Br- The acid catalyzed addition of water to an alkene produces an alcohol. When the same reaction is performed on an alkyne the product is called an enol. In most cases the enol is unstable compared to the keto form, the form with a C-C single bond and a C-O double bond. The conversion of the two forms is fast and is an equilibrium process. Once a hydrogen has been replaced by a halogen substitution can occur: Substitution is a reaction where one substituent substitutes for another. Two important types of substitution reaction are SN1 and SN2. SN1 stands for Substitution, Nucleophilic, 1st order. In this reaction a substituent leaves the substrate (it is called a leaving group). This generates a carbocation. The carbocation is the open to attack by a nucleophile. In this reaction, tertiary substrates will react faster than secondary, which are faster than primary. This is because more substituted carbocations are more stable. The reaction is first order because the first step, which involves only 1 molecule, is the rate determining step. - + - SN2 stands for Substitution, Nucleophilic, 2nd order. In this reaction a nucleophile attacks a substrate and displaces a substituent (the leaving group). Primary reacts faster than secondary which reacts faster than tertiary. This is because steric hindrance (blocking by atoms) slows the reaction down. It is a onestep reaction with two things involved so it’s second order. - - E1 is Elimination, 1st order. In this reaction a leaving group leaves as in SN1. Instead of a nucleophile bonding with the carbocation a base takes a hydrogen from a carbon attached to a carbon adjacent to the carbocation. The electrons from this bond go to make a double bond between the carbon formerly attached to the hydrogen and the carbon with the carbocation carbon. What has happened in the overall reaction is that the substrate lost a hydrogen and a leaving group and gained a double bond. The slow step is the formation of the carbocation which makes the reaction 1st order. + Br- B E2 is Elimination, 2nd order. In this reaction a base takes a hydrogen adjacent to a carbon with a leaving group. The electrons from the bond between the C and the H make a double bond between the two carbons of interest. It is a one-step reaction with two things involved so it’s second order. B BH+ Br- Aromatic compounds have cyclic structures with conjugated pi bonds. Conjugated pi systems are systems with alternating single and double bonds. The number of electrons in the system must satisfy the equation 4n+2. Aromatic compounds are more stable than other alkenes and their reactivity is different. Aromatic compounds are not normally involved in addition reactions. It is also more difficult to hydrogenate aromatic alkenes. Higher pressure and/or more powerful catalysts must be used compared to non-aromatic alkenes. Aromatic compounds can react in a way we haven't seen yet. In electrophilic aromatic substitution an electrophile substitutes for a hydrogen on an aromatic ring. HNO3 H2SO4 SO3 H2SO4 R AlCl3 RX R AlCl3 R Br2 FeBr3 The bromine is the electrophile, a species that "likes" electrons. The ferric bromide is a catalyst. Let's look at the mechanism: - - HBr Note that the mechanism left out the FeBr3 catalyst. The catalyst works when one of the bromine atoms in Br2 coordinates to the iron metal, weakening the Br-Br bond. As you can see from the mechanism in order for the reaction to happen the aromatic ring must send electrons to the electrophile. This explains the order of reactivity for substituted benzenes. It also explains the regioselectvity of reactions of substituted benzenes. Let's look at the bromination of phenol, benzene, and nitrobenzene: Br2 FeBr3 Br2 FeBr3 Br2 FeBr3 Note that the benzene can add the bromine to any one of the 6 positions with equal probability. The penol will add bromine to the ortho (adjacent) and para (180 degrees) positions while the nitrobenzene adds to the meta (120 degrees) position. A very small amount of bromine can add to the other positions of phenol and nitrobenzene just because of random molecular motion and statistical mechanics. Let's look at some resonance forms of phenol and nitrobenzene: : : : .. : : .. .. : : .. .. Note that a - charge is placed at the ortho and para positions. Note that a + charge is placed at the ortho and para positions. The reaction is called electrophilic aromatic substitution and that for it to occur the aromatic ring must donate electrons to the electrophile. In phenol, the oxygen donates electron density into the ring but in nitrobenzene the nitro group removes electron density from the ring. Therefore, phenol is more reactive to EAS than benzene, and benzene is more reactive than nitrobenzene. What if we have two groups? In that case if both groups are directing to the same position it is easy to predict where the substitutuion should occur. If the two gropus are directing to different positions go with the strongest activating group. OH- Carbonyls The carbonyl group is commonly found in organic compounds. It is comprised of a crbon double bonded to oxygen. If the carbon is bonded to two other carbons the group is also a ketone. One carbon and one hydrogen is an aldehyde. Two hydrogens is the molecule methanal, also called formaldehyde. One carbon and an oxygen bonded to hydrogen is a carboxylic acid. One carbon and an oxygen bonded to another carbon is an ester. One carbon and a halide is an acid halide. One carbon and a nitrogen is an amide. An acid anhydride has this structure: A reaction we'll look at is saponification. This is the process by which soap is made. Animal fat or vegetable oil is boiled in aqueous base. This hydrolyzes the ester bonds of the fat/oil and produces glycerol which is removed. It also produces free fatty acids that are deprotonated by the base. OHH2O - - Glycerol Fischer esterification is a name reaction, a reaction named after a scientist involved in developing it. In Fischer esterification a drop of acid, usually sulfuric catalyzes the formation of an ester from an alcohol and a carboxylic acid. Spectroscopy Mass spectrometry: Mass spec allows us to see what parts a molecule is made of. It works by ionizing the molecule to a cation and sending it through a magnetic field: You can see that the mass/charge ratio will determine how much the ion bends and what its path will be. This is how different m/z ratios are detected. Peaks to know: Alkanes: peaks that are 14 m/z apart Alkenes: 14 apart, C5H9+, C4H7+, C3H5+ Aromatic: massive base peak, 91 = C6H5CH2+ Alcohol: Primary: CH2OH+ = 31, then 45, 59,73…. Ketone: 43, 57, 71… Aldehyde: 29, 43, 57, 71 Amine: 30, 44, 58 Infrared spectroscopy: IR shows us which bonds are present in a molecule It works by determining how much IR radiation of each wavelength can be transmitted through a sample of molecule Bands to know: Alkane: C-H stretch 2840-3000, methyl bend at 1375, 1450 Alkene: C-H stretch 3000-3100 Alkyne: C-H stretch 3270-3300, C-C stretch at 2260-2100 (medium intensity) Alcohol: O-H stretch at 3650-3580, often the peak is broad due to H-bonding C-O stretch 1000-1100 Carbonyl: Strong sharp peak at 1540-1870 Amine: N-H stretch 3250-3500, weaker than O-H Nuclear Magnetic Resonance (NMR) http://www.chem.unc.edu/courses/550L/nmr_tutorial/index.html NMR is based upon the fact that some nuclei have spin. If they do, they act like little magnets. If we put these nuclei in a magnetic field the spins align parallel and antiparallel to the field. There is a quantized energy difference between the two sates. The difference in energy corresponds to radio in the electromagnetic spectrum. Sending a pulse of radio frequency energy into the sample causes some of the spins to flip. When the spins flip back they emit radio energy that can be detected which is the signal for the machine. Chemical shift: All nuclei of the same nuclide will have the same energy difference between parallel and antiparallel if they are in the same environment. Luckily for chemists the environment of the nucleus varies depending on the structure of the molecule. This is called chemical shift. We assign the hydrogens in tetramethylsilane a value of 0 for their chemical shift: On the other hand fluoromethane’s hydrogens have a chemical shift of 4.30. We can say that when there is more electron density around a nucleus the chemical shift is lower and when there is less electron density around the nucleus the chemical shift is higher. We say that the nuclei are shielded and deshielded, respectively. Since we are studying chemistry there are some interesting exceptions to this general rule. For example, alkenes generally have shifts around 5.4 while ethyne is 1.8. Benzene is 7.3. This is due to diamagnetic anisotropy which means that moving electrons generate magnetic fields. These fields can shield or deshield nuclei depending on their orientation. An extreme example of this is annulune which has 1 peak at 8.9 and another at -1.8: Coupling/splitting The proton’s spin can couple to the spin of another proton. This changes the energy of the proton. Identical protons do not couple so if we look at a fragment of a molecule CHCH2 we would see two peaks because there are two different protons in the fragment. The single proton can interact with both of the two protons on the other carbon. There are two possible interactions, the coupling can lower the energy of the proton or raise it. So, the CH2 protons show up as two peaks instead of one. We call this a doublet. The CH2 protons can couple to the CH proton and several things can happen. One is to have both CH2 protons lowering the energy of the CH, another is both raising it. Or one can raise it and one can lower it which cancels out. This creates a triplet where the middle peak is twice as high as the two peaks beside it. With just the information we’ve learned so far about chemical shift and splitting we can solve many structures. Here’s a few examples to help with inductive reasoning so we can apply deductive reasoning to solving structures later: NMR for Ethylbenzene Integration: This one is easy. The NMR spectrometer can integrate the area under the peaks to find the relative number of protons in a peak.