Chemistry: Homework Set 1-5
advertisement

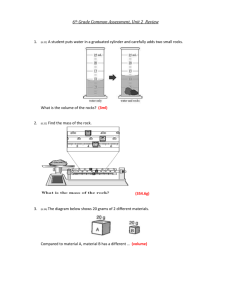
Chemistry: Homework Set 1-5 1. Three students were asked to determine the volume of a liquid by a method of their choosing. Each student performed three trials. The table below shows the results. The actual volume of the liquid is 24.8 mL. Trial #1 (mL) Trial #2 (mL) Trial #3 (mL) Student A 24.9 24.6 24.8 Student B 18.5 18.7 18.4 Student C 22.3 24.4 25.9 a. Which student’s measurements show good precision but poor accuracy? b. Which student’s measurements show both good precision and good accuracy? c. Determine Student C’s percent error in trial #1. 2. A student wants to determine the density of a rock. The rock, which has a mass of 34.89 grams, was lowered into a graduated cylinder holding 36.4 mL of water. The height of the water rose to 42.8 mL after the rock was placed in the cylinder. a. Based on the information given, calculate the density of the rock. b. If the actual density of the rock is 5.92 g/mL, determine the student’s percent error. Chemistry: Homework Set 1-5 1. Three students were asked to determine the volume of a liquid by a method of their choosing. Each student performed three trials. The table below shows the results. The actual volume of the liquid is 24.8 mL. Trial #1 (mL) Trial #2 (mL) Trial #3 (mL) Student A 24.9 24.6 24.8 Student B 18.5 18.7 18.4 Student C 22.3 24.4 25.9 a. Which student’s measurements show good precision but poor accuracy? b. Which student’s measurements show both good precision and good accuracy? c. Determine Student C’s percent error in trial #1. 2. A student wants to determine the density of a rock. The rock, which has a mass of 34.89 grams, was lowered into a graduated cylinder holding 36.4 mL of water. The height of the water rose to 42.8 mL after the rock was placed in the cylinder. a. Based on the information given, calculate the density of the rock. b. If the actual density of the rock is 5.92 g/mL, determine the student’s percent error.