Metode şi tehnici de studiu a suprafeţelor
advertisement
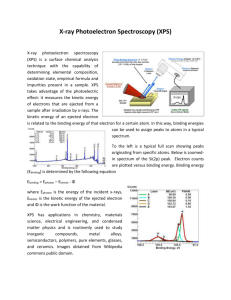
Methods and Tehniques in Surface Science Prof. Dumitru LUCA “Alexandru Ion Cuza” University, Iasi, Romania Historic 1887 – Discovery of the photoelectric effect (PE): Heinrich Hertz 1895 – Discovery of X-rays: Wilhelm Conrad Roentgen 1901 – Awarded (the FIRST) Nobel prize. H. Hertz 1905 – Explanation of the PE by Albert Einstein. 1921 – Awarded Nobel prize. Karl Manne Georg Siegbahn, (1886 – 1978) Univ. of Upsala, Sweden. Nobel prize in 1924 for his results in X-ray spectroscopy. W.C. Roentgen A. Einstein Kai M. Siegbahn (SON!) (1918 - ). Nobel prize in 1981 for his discoveries in high-resolution electron spectroscopy. 1950’s – huge progress in instrumentation: - increasing the resolution of the photoelectron energy analysers, - design of X-ray sources. - application for surface analysis (named ESCA) by K. Siegbahn and co-workers - also now - the discovery of the PE in gases (Turner and coworkers) and the development of the UPS (Spicer and co-workers) 1960: occurrence of commercial XPS instruments. Hamamatsu, November 2007 K.M.G. Siegbahn Kai M. Siegbahn What information can be derived from the XPS spectra? The most frequently used experimental technique in Surface Science to extract information on: The relative chemical composition in the surface region, The chemical status of elements, The dispersion of some phases within other phases, The depth profile of chemical composition, Valence band level structure. The “universal” curve: the dependence of the inelastic mean free path inel of photoelectrons, on their energy. Hamamatsu, November 2007 Electron spectroscopies Auger Electron Spectroscopy X-ray Electron Spectroscopy XPS AES X-rays or electrons X-rays Ultraviolet Electron Spectroscopy UPS UV photons Vac Vac V 3s EL2,3 2p6 EL1 2s2 EK 1s2 KE = EK-EL1-EL23- KE = h-EL1- Hamamatsu, November 2007 KE = h-EV- XPS vs. UPS 1. In XPS, holes are excited by X rays within the core level of the atom to derive the binding energy of the electrons in the lower lewels. After a core-level electron absorbs (integrally!) the energy of the X-ray photon, it leaves the atom, thus becoming a photo-electron: KE = h – Eb – Er - - δE ≈ h – Eb – , 2. In UPS, similar phenomena occur, except for UV potons are used here. The photo-electrons originate now in the valence band of the element(s). Mg Kα- 1253,6 eV Al Ka = 1486.6 eV Cu Ka = 8047 eV Fwhm= 0.75 eV Fwhm= 0.95 eV Fwhm= 2.6 eV Hamamatsu, November 2007 KE→ BE Mg K 330 eV 910 eV 920 eV 690 eV 720 eV 581 eV Transformarea (KE) in EB (BE = h KE) 920 eV 534 eV 561 eV 343 eV 333 eV 54, 88 eV (4s, 4p) 673 eV 0-8 (4-12) eV (4d, 5s) Hamamatsu, November 2007 Intensity N(E) Ecin = hν - EB 0 eV Energie de legatura, EB (eV) EF Nivele adanci Banda de valenta Banda de conductie http://www.nottingham.ac.uk/~ppzpjm/sect6_1. htm Hamamatsu, November 2007 Spin-orbital splitting Hamamatsu, November 2007 BE-Z Hamamatsu, November 2007 Chemical shift: wat is it and why does it occur? The charge transfer between neighboring atoms results in the alterations of the binding energies of the atom. Li-metal 1s2 1s2 1s2 A core level electron “feels” the nucleus more strongly than a valence electron (due to the differences in dimensions of the two types of orbitals). Thus, the electrostatic potential created by valence electrons as experienced by a core level electron is q/rv. 2s density Li: 1s2 2s1 O: 1s2 2s2 2p4 Li2O 2p6 2s 1s2 2s 1s2 2s2 Li O By losing a valence electron, the BE of the electron in a core-level of the atom becomes larger. 1 s2 Li Li2O Li-metal Binding Energy Hamamatsu, November 2007 EF 0 Chemical shift 2.1 eV 4.3 eV The BE values are affected not only by the energy levels, specific for any element. The BE values depend (to a lesser extent) on the chemical state of that element, since the core-level electrons are affected by the chemical state of the atom. Usually, chemical shift values are ranging between 1 and 5 eV. Hamamatsu, November 2007 Instrumentation Hamamatsu, November 2007 The PHI VersaProbe 5000 XPS machine View of the XPS machine in our lab with the following options: (a) XPS with chemical imaging; (b) AES; (c) Depth profiling; (d) UPS (future development) Hamamatsu, November 2007 Quantitative analysis using XPS: Relative elemental composition Ii=Fx i(EK) ni i(Ek) K cos θ where Ii – intensity of the p - peak, corresponding to the element i, I – ionisation cross-section (Scofield factor) of the element i (values calculated and tabulated for all the elements,including Al K and Mg K) ni – average concentration of the element i in the surface region, I – mean free path for the inelastic collision of a photoelectron of the element I, K – all the remaining factors involved in the detection of the photoelectrons, θ – take-off angle of photoelectrons. Typical accuracy: 10% Hamamatsu, November 2007 Background subtraction step background linear background Shirley background [D.A. Shirley, Phys. Rev. B5, 4709, 1972] Hamamatsu, November 2007 An example: the atomic percentage in a catalyst calculated from peak areas VPO Catalyst Element Peak area (arb. units) ASF Percentage (%) Carbon 1853 0.319 22.1 Oxygen 14240 0.75 62.0 Vanadium 3840 2.0 6.3 Phosphor 1494 0.64 9.6 Atomic Percent = Area1Sample ASF 1Sample N Area X Sample 1 ASF X Sample Hamamatsu, November 2007 Conclusion The main features of the XPS: Chemical identification: all the elements except for H and He, Probing depth: 1 – 6 nm, Detection limit: 0.1%, Determine the atomic environment and the oxidation state, Determine the depth-profile of the elemental concentration, Information about surface electrical properties from surface electrical charging, Lateral resolution: tens of micrometers; Energy resolution in BE: 10 meV. Hamamatsu, November 2007 Further references 1. D. Briggs, M. P. Seah, Practical surface analysis, vol. I, Willey and Sons, 1990. 2. J. M. Walls, R. Smith, Surface Science Techniques, Pergamon, 1994. 3. H. Lüth, Surfaces and interfaces of solid materials, Springer, 1993. 4. J. W. Niemantsverdriet, Spectroscopy in Catalysis – An Introduction, Wiley-VCH, 1995. 5. http://www.chem.qmul.ac.uk/surfaces/scc/scat5_3.htm 6. C.D. Wagner, W.M. Riggs, L.E. Davis, J.F. Moulter, G.E. Muilenberg, Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corp. (1978). 7. C.D. Wagner, Practical Surface Analysis, Vol. 1, 2ª, J. Wiley and Sons, 1990. 8. W.N. Delgass, G.L. Haller, R. Kellerman, J.H. Lunsford, Spectroscopy in heterogeneous catalysis, Cap. 8: X-ray Photoelectron Spectroscopy, Academic Press (1979). 9. H.D. Hagstrum, J.E. Rowe, J.C. Tracy, Electron spectroscopy of solid surfaces, in Experimental methods in catalytic research, Vol. 3, R.B Anderson y P.T. Dawson (Ed.), Academic Press (1976). 10. C.D. Wagner, L.E. Davis, M.V. Zeller, J.A. Taylor, R.M. Raymond, L.H. Gale, Surf. Interf. Anal. 3 (1981) 21. (for atomic sensitivity factors). 11. Moulder, John F., William F. Stickle, Peter E. Sobol, and Kenneth D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, ed. Jill Chastain and Roger C. King Jr. 1995: Physical Electronics, Inc., USA. 11 12. http://seallabs.com/howes1.html 13. http://srdata.nist.gov/xps/elm_in_comp_res.asp?elm1=C Hamamatsu, November 2007