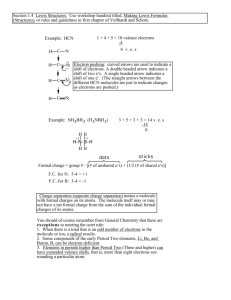
2 - Revsworld
advertisement
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; Bruce E. Bursten and Catherine J. Murphy Chapter 6 Electronic Structure of Atoms Donna Narsavage Heald Siena College Loudonville, NY 2009, Prentice Hall Considering only the n = 1 to n = 5 states in the hydrogen atom, which transition will emit the most energy? 1. 2. 3. 4. 5. n = 5 to n = 4 n = 5 to n = 2 n = 3 to n = 1 n = 3 to n = 2 n = 4 to n = 2 Considering only the n = 1 to n = 5 states in the hydrogen atom, which transition will emit the most energy? 1. 2. 3. 4. 5. n = 5 to n = 4 n = 5 to n = 2 n = 3 to n = 1 n = 3 to n = 2 n = 4 to n = 2 Considering only the n = 1 to n = 5 states in the hydrogen atom, which transition will emit the longest wavelength? 1. 2. 3. 4. 5. n = 5 to n = 4 n = 5 to n = 2 n = 3 to n = 1 n = 3 to n = 2 n = 4 to n = 2 Considering only the n = 1 to n = 5 states in the hydrogen atom, which transition will emit the longest wavelength? 1. 2. 3. 4. 5. n = 5 to n = 4 n = 5 to n = 2 n = 3 to n = 1 n = 3 to n = 2 n = 4 to n = 2 What are the valence electrons of vanadium? 1. 2. 3. 4. 5. 4s2 3d3 4s23d3 3d5 4d5 What are the valence electrons of vanadium? 1. 2. 3. 4. 5. 4s2 3d3 4s23d3 3d5 4d5 What are the valence electrons of gallium? 1. 2. 3. 4. 5. 4s2 4p1 4s23d104p1 4s23d10 4s24p1 What are the valence electrons of gallium? 1. 2. 3. 4. 5. 4s2 4p1 4s23d104p1 4s23d10 4s24p1 What is the maximum number of orbitals described by the quantum numbers: n=3 1. 2. 3. 4. 5. 1 3 5 8 9 l=2 What is the maximum number of orbitals described by the quantum numbers: n=3 1. 2. 3. 4. 5. 1 3 5 8 9 l=2 What is the maximum number of electrons described by the quantum numbers: n=4 1. 2. 3. 4. 5. 7 14 16 32 48 What is the maximum number of electrons described by the quantum numbers: n=4 1. 2. 3. 4. 5. 7 14 16 32 48 How many nodal planes does a d orbital have? 1. 2. 3. 4. 5. 0 1 2 3 4 How many nodal planes does a d orbital have? 1. 2. 3. 4. 5. 0 1 2 3 4 How many unpaired electrons does selenium have? 1. 2. 3. 4. 5. 0 2 4 6 8 How many unpaired electrons does selenium have? 1. 2. 3. 4. 5. 0 2 4 6 8 How many unpaired electrons does chromium have? 1. 2. 3. 4. 5. 0 2 4 6 8 How many unpaired electrons does chromium have? 1. 2. 3. 4. 5. 0 2 4 6 8