CFT
advertisement
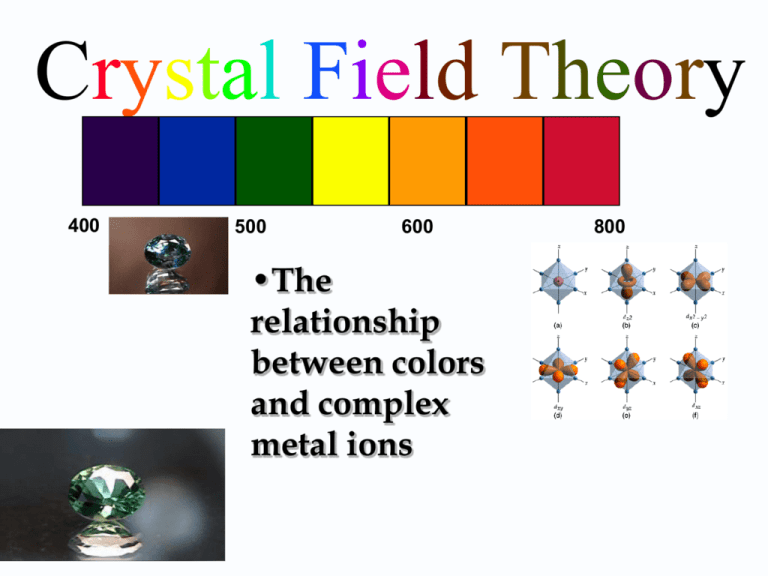
Crystal Field Theory 400 500 600 •The relationship between colors and complex metal ions 800 Crystal Field Model A purely ionic model for transition metal complexes. Ligands are considered as point charge. Predicts the pattern of splitting of d-orbitals. Used to rationalize spectroscopic and magnetic properties. Salient features of CFT The interaction between the metal ion and the ligand is electrostatic one i.e ionic. The metal ion the ligands are considered as point charges. The negative ligands are regarded as negative point charges and the neutral ligands are regarded as diploes. The negative end of the ligand dipole is oriented towards the metal ion. The central metal ion is surrounded by ligands that contain one or more lone pair of electrons. The lignand electron pairs can’t enter into the metal orbitals. Thus there is no orbital overlap between the metal and the ligand. Number and the nature of lignds and their arrangement around the central metal ion will determine the crystal field Different crystal field will have different effects on the relative energies of the five d orbitals. The electrons of the central metal ion and those of the ligands have repulsive effect. This causes the splitting of the degenerate d orbitals into two groups- namely t2g and eg. It is called crystal field splitting. d-orbitals: look attentively along the axis Linear combination of dz2-dx2 and dz2-dy2 d2z2-x2-y2 Octahedral Field 19.3 Crystal Field Theory: Splitting of the 5 d orbitals Consider the response of the energy of the d orbitals to the approach of 6 negatively charged ligands (a “crystal field”) along the x, y and z axes of the metal The two d orbitals (dx2-y2 and dz2) that are directed along the x, y and z axes are affected more than the other three d orbitals (dxy, dxz and dyz) eg orbitals crystal field energy splitting The result is that the dx2-y2 and dz2 orbital increase in energy relative to the dxy, dxz and dyz orbitals (D0 is called the “crystal field energy splitting” t2g orbitals 7 Crystal field splitting of the 5 d orbitals by the “crystal field” of 6 ligands eg orbitals Crystal field splitting t2g orbitals Orbitals “on axis”: “energy increases” Orbitals “off axis”: “energy decreases” 8 Crystal Field Splitting Energy (CFSE) • In Octahedral field, configuration is: t2gx egy • Net energy of the configuration relative to the average energy of the orbitals is: = (-0.4x + 0.6y)O O = 10 Dq BEYOND d3 • In weak field: O P, => t2g3eg1 • In strong field O P, => t2g4 • P - paring energy The Spectrochemical Series Based on measurements for a given metal ion, the following series has been developed: I-<Br-<S2-<Cl-<NO3-<N3-<F-<OH-<C2O42-<H2O <NCS-<CH3CN<pyridine<NH3<en<bipy<phen <NO2-<PPh3<CN-<CO The Spectrochemical Series The complexes of cobalt (III) show the shift in color due to the ligand. (a) CN–, (b) NO2–, (c) phen, (d) en, (e) NH3, (f) gly, (g) H2O, (h) ox2– , (i) CO3 2–. Tetrahedral Complexes Square Planar Complexes Ligand Field Strength Observations 1. ∆o increases with increasing oxidation number on the metal. Mn+2<Ni+2<Co+2<Fe+2<V+2<Fe+3<Co+3 <Mn+4<Mo+3<Rh+3<Ru+3<Pd+4<Ir+3<Pt+4 2. ∆o increases with increases going down a group of metals. Magnitude of Oxidation state of the metal ion [Ru(H2O)6]2+ 19800 cm-1 [Ru(H2O)6]3+ 28600 cm-1 Ground-state Electronic Configuration, Magnetic Properties and Colour When the 4th electron is assigned it will either go into the higher energy eg orbital at an energy cost of o or be paired at an energy cost of P, the pairing energy. d4 Strong field = Low spin (2 unpaired) P < o P > o Coulombic repulsion energy and exchange energy Weak field = High spin (4 unpaired) Ground-state Electronic Configuration, Magnetic Properties and Colour [Mn(H2O)6]3+ Weak Field Complex the total spin is 4 ½ = 2 High Spin Complex [Mn(CN)6]3Strong field Complex total spin is 2 ½ = 1 Low Spin Complex What is the CFSE of [Fe(CN)6]3-? C.N. = 6 Oh Fe(III) d5 3- CN NC CN- = s.f.l. h.s. l.s. eg CN eg + 0.6 oct Fe CN NC CN - 0.4 oct t2g t2g CFSE = 5 x - 0.4 oct + 2P = - 2.0 oct + 2P If the CFSE of [Co(H2O)6]2+ is -0.8 oct, what spin state is it in? C.N. = 6 Oh Co(II) d7 2+ OH 2 H 2O h.s. l.s. eg eg + 0.6 oct OH 2 Co OH 2 H 2O OH 2 t2g CFSE = (5 x - 0.4 oct) + (2 x 0.6 oct) +2P = - 0.8 oct+2P t2g - 0.4 oct CFSE = (6 x - 0.4 oct) + (0.6 oct) + 3P= - 1.8 oct + P The origin of the color of the transition metal compounds E2 E h E1 E = E2 – E1 = h Ligands influence O, therefore the colour The colour can change depending on a number of factors e.g. 1. Metal charge 2. Ligand strength The optical absorption spectrum of [Ti(H2O)6]3+ Assigned transition: eg t2g This corresponds to the energy gap O = 243 kJ mol-1 absorbed color observed color • Spectrochemical Series: An order of ligand field strength based on experiment: Weak Field I- Br- S2- SCN- Cl- NO3- F- C2O42- H2O NCS- CH3CN NH3 en bipy phen NO2- PPh3 CN- CO Strong Field H2 N NH2 N N N N Ethylenediamine (en) 2,2'-bipyridine (bipy) 1.10 - penanthroline (phen) [CrF6]3- [Cr(H2O)6]3+ [Cr(NH3)6]3+ [Cr(CN)6]3- As Cr3+ goes from being attached to a weak field ligand to a strong field ligand, increases and the color of the complex changes from green to yellow. Limitations of CFT Considers Ligand as Point charge/dipole only Does not take into account of the overlap of ligand and metal orbitals Consequence e.g. Fails to explain why CO is stronger ligand than CN- in complexes having metal in low oxidation state