Biochemistry - Chemistry of life note packet
advertisement

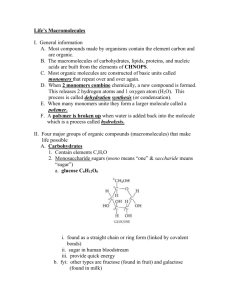
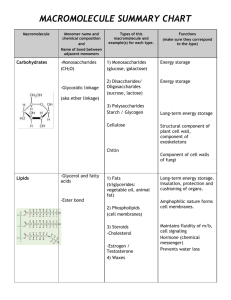
Bio H – Biochem – Unit 3 Biochemistry - Chemistry of life note packet The previous unit covered primarily inorganic chemistry. But, most of the key biological molecules are organic. Inorganic molecule: ______________________________________________________________________________ - Examples: notice some may have carbon OR hydrogen, just not both Organic molecule: _________________________________________________________________________________ - Hydrocarbons: _________________________________________________________________________________ Examples: Which of the following organic molecules are ALSO hydrocarbons? This unit will cover the structure/composition and function of all the major compounds needed by the cell. I. Water – inorganic, but very important. In the last unit we discussed the chemical properties of water, now we will address its many functions within living things Water makes up 65-75% of the body and an even higher percentage of the average cell. Below is a list of functions. Describe how/where each occurs from our discussions in class. In the body water acts as a: 1. Solvent: ___________________________________________________________________________ 2. Medium: ___________________________________________________________________________ 3. Fluid to keep surfaces moist: ___________________________________________________________________________ 4. Temperature regulator: ___________________________________________________________________________ 5. Cushion: ___________________________________________________________________________ 1 Bio H – Biochem – Unit 3 6. Transportation fluid: ___________________________________________________________________________ 7. Lubricant: ___________________________________________________________________________ 8. Reactant for hydrolysis: ___________________________________________________________________________ 9. Necessary assistant to our sense organs (taste, smell, sight) : ___________________________________________________________________________ II. Minerals: basic elements that are needed by our body. Include the minerals we call “electrolytes.” We can also refer to these as “ions” A. General Functions of minerals: a. ________________________________________________________________ b. _________________________________________________________________ c. _________________________________________________________________ d. _________________________________________________________________ B. Examples of minerals and where they work a. Iron: ______________________________________________________________ b. Sodium, Chlorine, potassium: _____________________________________________________ ____________________________________________________________________________ c. Calcium: ______________________________________________________________ d. Iodine: ______________________________________________________________ e. Phosphorous: ______________________________________________________________ 2 Bio H – Biochem – Unit 3 MAJOR ORGANIC MOLECULES (except for water, the most prevalent) IN THE BODY The remaining four compounds of the cell are called Macromolecules, meaning ______________________________. - carbohydrates, proteins and nucleic acids are all made of small molecules called _________________________ That can be linked together into chains called __________________________. STRUCTURE OF MACROMOLECULES BUT there are many types of monomers and each class of compounds has its own unique monomer. 1. ALL MACROMOLECULES HAVE A CARBON BACKBONE! _____________________ is an incredibly important element. Without it, we would not be able to build the millions of different types of molecules. Why is it so important? What different types of things can it do? • Since Biochemistry uses so many C’s and H’s, we can simplify our structures by “hiding” some of them There is a Carbon atom at each corner or point It is also assumed that, if no atom is shown, Carbons remaining bonds are filled up with H Draw some examples from the board: 3 Bio H – Biochem – Unit 3 Synthesis and breakdown of macromolecules into polymers: a. The body uses the same chemical reaction to build all the major classes of compounds (dehydration synthesis or a condensation reaction) AND the same reaction to break them all down (hydrolysis). - A .Dehydration synthesis (Condensation) : ___________________________________________________________________________ _______________________________________________________________________________________________ o Step 1: begin with at least two unlinked monomers. The middles of our monomers may be different and are represented by the blob with an X in it. BUT all the monomers we’ll be dealing with have the same ends and that is what is important for linking them. HO X OH + HO X OH Reactants: Two monomers o Step 2: Remove an H from monomer 1 and an OH from monomer 2; The H and OH combine to form water HO HO OH O + + HO OH OH HOH = H2O o Step 3: connect what is left of the monomers; HO o O OH + H2O Products: Dimer (two connected monomers) and a water molecule Step 4: If need be, you can repeat the reaction many more times adding monomers to your growing chain. 4 Bio H – Biochem – Unit 3 - B. Hydrolysis: _____________________________________________________________________________________ _______________________________________________________________________________________________ o Step 1: Begin with a polymer and 1 water molecule HO o OH + H2O Step 2: Separate the H2O into an H and an OH. - Attach the H to one side of the bond, attach the OH to the other OH O H HO HO o O Final product of Hydrolysis: two separate monomers HO OH HO OH TAKE A BREATH…..We have learned a lot in the last 5 pages. Take a minute to summarize 1-3 key points from each page (key points ONLY!) 5 Bio H – Biochem – Unit 3 NOW THAT WE UNDERSTAND HOW MACROMOLECULES ARE FORMED AND BROKEN DOWN, LETS LOOK AT EACH MACROMOLECULE I. Carbohydrates:(sugars, starches, cellulose, glycogen, chitin, monosaccharides, disaccharides, polysaccharides) There are three variations of a carbohydrate formula: 1. Molecular formula: just the atoms and their numbers; for example: _______________________________ 2. Linear or straight chain: Draw below 3. Structural: ring shaped; drawn as a hexagon or pentagon depending on the number of carbons. See below. Types of carbohydrates (based on size and complexity) 1. __________________________________ are the monomers of carbohydrates o These are the simple sugars. A single ring; o Made of C, H and O in a __________________________ratio o Examples include: _______________________________________________________________________ o What elements compose these carbohydrates?_________________________________ 2. Dissaccharide: Name means: _______________________________________________ 6 Bio H – Biochem – Unit 3 - Examples: 3. Polysaccharide: Name means:____________________________________________________________________________ - Examples: HOW DO WE USE DEHYDRATION SYNTHESIS TO FORM DI- and POLYSACCHARIDES FROM MONOSACCHARIDES? DEHYDRATION SYNTHESIS OF CARBOHYDRATES Below is the dehydration synthesis reaction of two monosaccharides into a disaccharide. - Highlight the atoms that are removed and ultimately become the water molecule + + H2O Give three examples of molecules that fit into this category: 1. 2. 3. FUNCTION OF CARBOHYDRATES (function depends on the form in the body)? FUNCTION 1 __________________________________ a. Monosaccharides: _______________________________ b. Polysaccharides: _________________________________ EXAMPLES Plant “ST energy” Polysaccharide is: Animal “ ST energy” Polysaccharide is: FUNCTION 2 _______________________________________________________________________________ EXAMPLES –plants structural polysaccharide: In Animals structural PS (bugs, lobsters, shrimp): 7 Bio H – Biochem – Unit 3 II. Lipids Do lipids have a true monomer? ___________ Why? ____________________________________________________ One type of lipid molecule is called a _________________________________________________ What three parts are linked together to form a triglyceride? _____________________________________________ Below is a picture of the dehydration synthesis of a glycerol molecule and three fatty acids: - highlight the atoms that become the water molecules. Then fill in how many waters are made H + + H __H2O H Three Fatty Acids 1 Triglyceride What elements compose this macromolecule:_________________________________ See your reading/questions on lipids for the following terms and their significance: - saturated vs. unsaturated fatty acids, hydrogenated fatty acids, trans fatty acids List examples of molecules are lipids: Steroids like cholesterol have a different structure and are built differently. Be able to recognize the structure below. List the important role that these molecules play in the body? 8 Bio H – Biochem – Unit 3 III. Proteins What is the monomer that makes up this macromolecule:____________________________ Below is a dehydration synthesis reaction of two amino acids: - highlight the atoms that become the water molecule, label the amino and carboxyl ends and the peptide bond + + H2O How many different types of Amino acids exist? _________ What part makes each one different? _______________________________ Which parts do each of them have in common? _______________________________ What elements compose Proteins?_________________________________ Once amino acids are linked in a chain, they are called a polypeptide. What has to happen to the polypeptide before it becomes a functional protein? There are 4 levels of protein structure: (see picture on next page) Primary – just the straight chain of amino acids Secondary – chain of amino acids twists or folds into shapes called Alpha – helices or Beta sheets Tertiary –secondary chain folds and wraps up into 3D “blob” due to interactions between amino acid side chains Quaternary – Multiple polypeptides, each folded into their own tertiary structure, interact together 9 Bio H – Biochem – Unit 3 Give examples of molecules that fit into this category and their function: 1. ________________________________________________________________________________________ 2. ________________________________________________________________________________________ 3. ________________________________________________________________________________________ 4. ________________________________________________________________________________________ 10 Bio H – Biochem – Unit 3 IV. Nucleic Acids What is the monomer that makes up this macromolecule:____________________________ Draw the structure of one monomer and label the THREE parts; include the variations: What elements compose this macromolecule:_________________________________ What is the general role for all nucleic acids: _________________________________________________________ ________________________________________________________________________________________ Compare and contrast the two types of Nucleic acids in the chart below. RNA DNA Full Name Sugar Possible Bases # of nucleotide chains in helix Function Location Is made in the nucleus, but quickly moves to ribosomes Nucleus Chains of nucleotides are also build and broken down via Dehydration synthesis and hydrolysis, BUT the structures are a little more complicated. You will not be asked to draw these reactions for Nucleic acids but WILL be expected to draw them for the other three classes of compounds discussed in this unit. 11