Lh6Ch06bEnzymes
advertisement

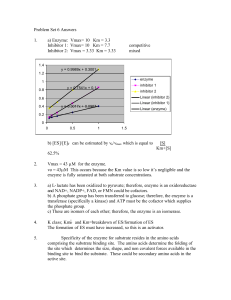
Chapter 6 Protein Function: Enzymes Part 2 Enzymes, Part 2 Learning Goals: To Know – Chemical mechanisms of catalysis: Chymotrypsin – Multi-substrate enzyme reaction kinetics – Reversible enzyme inhibitors and inhibition kinetics More fun with Practicase – Regulation of enzyme activity. Part of Worked Example 6.1 An enzyme, happyase, is discovered to catalyze: SAD HAPPY It is known that the kcat is 600 s-1. When [Et] = 20 nM, and [SAD] = 40 μM, the reaction velocity (vo) is 9.6 μM/s. What is the KM? Solution combines the kcat equation and the MichaelisMenten equation. kcat = Vmax/ [Et] so: Vmax = kcat [Et] Substitute into Michaelis-Menten equation for Vmax: vo = [ Vmax (S)] / [Km + (S)] vo = [kcat [Et] (S)] / [Km + (S)] Answer: Km = 10μM So Far… … … it has just been single substrate: S + E ES E + P Now lets go to multiple substrates and multiple products (we will only go as much as 2 substrates 2 products): Enz A + B C + D Multiple Substrate Reactions Lineweaver Burke Plot – Enzymes forming Ternary Complexes – Ordered or Random Lineweaver Burke Plot – Enzymes Without Ternary Complexes Enzyme Inhibition Inhibitors are compounds that decrease enzyme’s activity •Irreversible inhibitors (inactivators) react with the enzyme • One inhibitor molecule can permanently shut off one enzyme molecule • They are often powerful toxins but also may be used as drugs •Reversible inhibitors bind to and can dissociate from the enzyme • They are often structural analogs of substrates or products • They are often used as drugs to slow down a specific enzyme •Reversible inhibitor can bind: • to the free enzyme and prevent the binding of the substrate • to the enzyme-substrate complex and prevent the reaction Reversible Inhibitors - Competitive Lineweaver Burke - Competitive Inhibition α = 1 + [I]/Ki -1/Km -1/αKm Reversible Inhibitors - Uncompetitive Lineweaver Burke: Uncompetitive Inhibition α’ = 1 + [I]/Ki’ Reversible Inhibitors – Mixed Inhibition Lineweaver Burke – Mixed Inhibition α = 1 + [I]/Ki α’ = 1 + [I]/Ki’ α’/Vmax - 1/Km - α’/αKm Shows Region of Inhibitor Effect Apparent Vmax or Apparent Km refers to y or x axis intercept only. The Next Slide is MUCH BETTER Calculation of Enzyme Constants Type of Inhibition X axis intercept Y axis intercept None -1/Km 1/Vmax Competitive -1/αKm 1/Vmax Uncompetitive - α’/Km α’/Vmax Mixed - α’/αKm α’/Vmax EOC Problem 12: Lets figure out what sort of inhibitor ibuprofen (active ingredient in Advil) is. Ouch!!! or Ahhhh! And, next there is our friend Practicase and inhibitors. Inhibition of Practicase [Studentose],mM vo, uninhibited vo Inhbitor A vo Inhibitor B vo Inhibitor C 1 12 4.3 5.5 2 20 8 9 8.69 4 29 14 13 13.7 8 35 21 16 19.6 12 40 26 18 22.2 Inhibitor A at 1 mM Inhibitor B at 3 mM Inhibitor C at 50 μM 5 L-B plots of Practicase Inhibitiors We already know KM = 3.33 mM and Vmax= 50 µmoles/mL/s and it is a Competitive Inhibitor Calculation Inhibitor A’s Practicase Ki : The Inhibited curve intersects the X-axis at 0.1 mM, thus -1/αKM = -0.1 mM Be sure to calculate the Ki’s for the other inhibitor. Is the inhibitor a potential drug? Compare the Ki to the KM…what does this tell you? Solving for α, α = 3 So, α = 1 + [ I ]/Ki we know the inhibitor in the experiment was 1 mM Thus, 3 = 1 + 1mM/Ki 2 = 1mM/Ki Ki = 0.5 mM Thus inhibitor A binds the enzyme BETTER than the substrate! Practicase Inhbitiors Calculation of Mixed Inhibitor’s Ki’s This is Inhibitor 2 This inhibitor has α and α’… to calculate Ki and Ki’ So, FIRST you need to calculate α’ … the best place to do that is from the y-axis intercept = α’/Vmax Then to get α, go to the x-axis intercept = α’/αKm then from each, α and α’ you can determine Ki and Ki’ Remember this one inhibitor binds both to E and ES. Enzymes and Fashion “Stonewashed Jeans” Jeans are washed with cellulase (an enzyme that hydrolyzes celluose – major component in cotton) at a low concentration for a short time…..the effect looks “stonewashed”. If they were really stonewashed how would they get all the stones out of the pockets? Irreversible Inhibition Mechanism Based Suicide Inhibitors Effect of pH on Enzyme Activity Effect of pH on Chymotrypsin Chymotrypsin – Our Model Enzyme Active Site of Chymotrypsin with Substrate Aromatic Part of Substrate = Green Chymotrypsin – Our Model Enzyme Amide Nitrogens Stabilize Oxyanion Reactive Groups in Enzymes are Either: Chymotrypsin Mechanism Step 1: Substrate Binding Chymotrypsin Mechanism Step 2: Nucleophilic Attack Chymotrypsin Mechanism Step 3: Substrate Cleavage Chymotrypsin Mechanism Step 4: Water Comes In Chymotrypsin Mechanism Step 5: Water Attacks Chymotrypsin Mechanism Step 6: Break-off from the Enzyme Chymotrypsin Mechanism Step 7: Product Dissociates Drug Company Recruiting Ad The importance of structural protein chemistry !!! And transition state analogs… Which bind the active site exceptionally well. from C&EN, Aug 13, 2007 Hexokinase Reaction : Induced Fit What happens when glucose binds Induced Fit with Glucose Binding Daniel Koshland 60’s Remember in Part 1 of this Chapter glucose prevented thermal destruction of hexokinase…EOC problem 4. Xylose is One Carbon Shorter than Glucose Xylose causes Hexokinase to become an ATPase When Xylose reacts with Hexokinase – it causes induced fit and Mg++ ATP binds… but xylose does not exclude water from the active site where the 6th carbon would be. Normally the Induced fit is the active form, and catalyses the phospho-transfer from ATP to glucose glucose-6phosphate + ADP, but when xylose is there: Xylose + H2O + ATP Xylose + ADP + Pi…. A futile use of ATP! Enzyme activity can be regulated • Regulation can be: – noncovalent modification – covalent modification – and either • irreversible • reversible Noncovalent Modification: Allosteric Regulators The kinetics of allosteric regulators differ from Michaelis-Menten kinetics. Allosteric Effectors – Bind to Allosteric Site Feedback Inhibition is the Classic Form of Allosteric Inhibition Allosteric Enzymes Often Have Sigmoid Kinetics Allosteric Positive and Negative Regulators: Affecting KM Allosteric Positive and Negative Regulators: Affecting the Vmax Enzyme Regulation by Covalent Modification Glycogen Synthase Regulation: Both Allosteric and Covalent From Ch 15 PP1: Protein Phosphatase-1 Zymogen Regulation Things to Know and Do Before Class 1. Know the chymotrypsin reaction and the concept of how enzymes participate in the reaction. 2. Kinetics of multisubstrate reactions. 3. Types and kinetics of reversible enzyme inhibitors. And, the importance of Ki. 4. Types enzyme regulation and their Michaelis Menten kinetics. 5. Be able to do EOC Problems 12, 18, 19.