Stoichiometry 1 Formulas and the Mole
advertisement

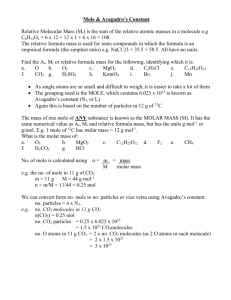
Stoichiometry 1 Formulas and the Mole L. Scheffler Lincoln High School 1 The Mole • Chemical reactions involve atoms and molecules. • The ratios with which elements combine depend on the number of atoms not on their mass. • The masses of atoms or molecules depend on the substance. • Individual atoms and molecules are extremely small. Hence a larger unit is appropriate for measuring quantities of matter. • A mole is equal to exactly the number of atoms in exactly 12.0000 grams of carbon 12. • This number is known as Avogadro’s number. 1 mole is equal to 6.022 x 1023 particles. 2 Definitions of the Mole • 1 mole of a substance has a mass equal to the formula mass in grams. • Examples • 1 mole H2O is the number of molecules in 18.015 g H2O • 1 mole H2 is the number of molecules in 2.016 g H2. • 1 mole of atoms has a mass equal to the atomic weight in grams. • 1 mole of particles = 6.02214 x 1023 particles for any substance! • The Molar mass is the mass of one mole of a substance • Avogadro's number is the number of particles (molecules) in one mole for any substance 3 The Mole 4 A mole is equivalent to a gram atomic weight or gram molecular weight The Formula Mass • The formula mass is the sum of atomic masses in a formula. • If the formula is a molecular formula, then the formula mass may also be called a molecular mass. 5 Gram Formula Mass and Molar Mass • If the formula mass is expressed in grams it is called a gram formula mass. • The gram formula mass is also known as the Molar Mass. • The molar mass is the number of grams necessary to make 1 mole of a substance. • The units for Molar Mass are g mol-1. 6 Formula Mass and the Mole • The atomic mass of Carbon 12 is exactly 12.00000. • 1 atomic mass unit = 1/12 of the atomic mass of carbon 12. • The periodic table gives the average atomic mass for an element relative to Carbon 12. • 1 mole of a substance is 6.022 x 1023 particles. • The mole of atomic mass units is equal to 1.000 gram. 7 Gram Formula Mass • The formula mass is the sum of the atomic masses in a formula. • A gram formula mass is the same number expressed in grams. • It is also equal to Avogadro’s Number of particles • Example: H2O From the Periodic Table - Atomic Masses: H =1.00797, O = 15.999 The formula mass = 2(1.00797)+15.999 = 18.015 • Adding the unit “grams” to the formula mass transforms it into a gram formula mass or mole. 8 The Mole • The mole is connects the macro world that we can measure with the micro world of atoms and molecules. • A Mole is also equal to – 1 gram formula mass. – 22.4 dm3 of any gas measured at 0o C and 1.0 atmosphere of pressure. 9 Example 1: Calculating the Molar Mass of a Compound • Calculate the gram formula mass or Molar Mass of Na3PO4. Atom Na P O # 3 1 4 Atomic Mass X 23.0 X 31.0 X 16.0 Total = = = = Total 69.0 31.0 64.0 164.0 Therefore the molar mass is 164.0 g mol-1 10 Example 2: Find the mass of 2.50 moles of Ca(OH)2 Find the molar mass of Calcium hydroxide and multiply by 2.50 mol The molar mass of Ca(OH)2 is 1 Ca 1 x 40.08 = 40.08 2 O 2 x 16.00 = 32.00 2 H 2 x 1.01 = 2.02 Molar Mass = 74.10 g mol-1 2.50 mol x 74.10 g mol-1 = 185.25 g 11 Calculating Moles • The number of moles in a given mass of a substance can be determined by dividing the mass by the molar mass Moles = Mass Molar Mass 12 Example 3: Find the number of moles in 44.46 grams of Ca(OH)2 Find the molar mass of and divide it into the given mass From the previous example the molar mass of calcium hydroxide is 74.10 gmol-1. 44.46 g Ca(OH)2 . = 0. 6000 mol 74.10 g mol-1 Ca(OH)2 13 Example 4: Calculating Moles • Calculate the number of moles in 20.5 grams of Na3PO4 Moles = Mass Molar Mass Moles = 20.5 g 164.0 g mol-1 = 0.125 mol Note: Mol is the standard abbreviation for a mole 14 Calculating Mass From Moles • The mass of a quantity of a substance can be found by multiplying the number of moles by the molar mass Mass = Moles X Molar Mass 15 Example 5 Calculating Mass from Moles • Calculate the mass of 2.50 moles of Na3PO4 Mass = Moles X Molar Mass = 2.50 mol x 164.0 g mol-1 = 409 g 16 Percentage Composition • According to the law of definite proportions, compounds, contain definite proportions of each element by mass. • The sum of all of the atomic masses of elements in a formula is called the formula mass. • If it is expressed in grams, then it is called a gram formula mass or molar mass. • If it represents the sum of all of the masses of all of the elements in a molecule then it is called a molecular mass. • To find the percentage of each element in a compound it is necessary to compare the total mass of each element with the formula mass. 17 Percentage Composition • The percent by mass of each element in a compound is equal to the percentage that its atomic mass is of the formula mass. • Example: Calculate the percentage of oxygen in potassium chlorate, KClO3 Atomic masses: K = 39.09, Cl = 35.45 and O = 16.00. Formula mass = 39.09 + 35.45+ 3(16.00) = 122.54 Percent Oxygen = (3(16.00)/122.54) (100) = 39.17% 18 Example 2 • Calculate the percentage by mass of each element in potassium carbonate, K2CO3 First calculate the formula mass for K2CO3 . Find the atomic mass of each element from the periodic table. Multiply it by the number of times it appears in the formula and add up the total 2 Potassium atoms K 1 carbon atom C 3 Oxygen atoms O 2 x 39.10 = 78.20 1 x 12.01 = 12.01 3 x 16.00 = 48.00 Total = 138.21 To find the percent of each element divide the part of the formula mass that pertains to that element with the total formula mass Percent of Potassium K = 78.20 X 100 138.21 =56.58 % Percent of Carbon C = 12.01 X 100 138.21 = 8.69 % Percent of Oxygen O = 48.00 = 34.73 % X 100 19 Empirical Formula Determination The empirical formula is the simplest ratio of the numbers of atoms of each element that make a compound. To find the empirical formula of a compound: 1. Divide the amount of each element (either in mass or percentage) by its atomic mass. This calculation gives you moles of atoms for each element that appears in the formula 2. Convert the results to small whole number ratios. Often the ratios are obvious. If they are not divide all of the other quotients by the smallest quotient 20 Example 1 Analysis of a certain compound showed that 32.356 grams of compound contained 0.883 grams of hydrogen, 10.497 grams of Carbon, and 27.968 grams of Oxygen. Calculate the empirical formula of the compound. First divide the amount by the atomic mass to get the number of moles of each kind of atom in the formula Hydrogen H = 0.883 g 1.01 g mol-1 = 0.874 mol Carbon C = 10.497 g 12.01 g mol-1 = 0.874 mol Oxygen O = 27.968 g 16.00 g mol-1 = 1.748 mol • Analysis of the ratio s shows that the first two are identical and that the third is twice the other two. Therefore the ratio of H to C to O is 1 to 1 to 2. The empirical formula is HCO2 21 Molecular Formula • To calculate the molecular formula from the empirical formula it is necessary to know the molecular (molar) mass. • Add up the atomic masses in the empirical formula to get the factor • Divide this number into the molecular formula mass. • If the number does not divide evenly you probably have a mistake in the empirical formula or its formula mass • Multiply each subscript in the empirical formula by the factor to get the molecular formula 22 Molecular Formula Example • Example: Suppose the molecular mass of the compound in the previous example, HCO2 is 90.0. Calculate the molecular formula. • The empirical formula mass of is 1H 1.0 x 1 = 1.0 1 C 12.0 x 1 = 12.0 2 O 16.0 x 2 = 32.0 Total 45.0 • Note that 45 is exactly half of the molecular mass of 90. • So the formula mass of HCO2 is exactly half of the molecular mass. Hence the molecular formula is double that of the empirical formula or H2C2O4 23 Part 2: Stoichiometry Problems • Mass-Mass Problems • Mass-Volume 24 Stoichiometry Problems • Stoichiometry problems involve the calculation of amounts of materials in a chemical reaction from known quantities in the same reaction • The substance whose amount is known is the given substance • The substance whose amount is to be determined is the required substance 25 Mass to Mass Problems • Goal: To calculate the mass of a substance that appears in a chemical reaction from the mass of a given substance in the same reaction. • The given substance is the substance whose mass is known. • The required substance is the substance whose mass is to be determined. 26 Steps in a Mass to Mass Problem 1. Find the gram formula masses for the given and the required substances 2. Convert the given mass to moles by dividing it by the molar mass 3. Multiple the given moles by the mole ratio to get the moles of the required substance 4. Multiple the number of moles of the required substance by its molar mass to get the mass of the required substance 27 Summary of Mass Relationships 28 Example 1 Mass-Mass Problem • Glucose burns in oxygen to form CO2 and H2O according to this equation: C6H12O6 + 6 O2 6 CO2 + 6 H2O How many grams of CO2 are produced from burning 45.0 g of glucose? 29 Example 1 Mass-Mass Problem Glucose burns in oxygen to form CO2 and H2O according to this equation: C6H12O6 + 6 O2 6 CO2 + 6 H2O How many grams of CO2 are produced from burning 45.0 g of glucose? 1.Make sure that the equations is balanced 2.Divide the mass of the given by its molar mass 45.0 g C6H12O6 x 180.0 g mol-1 C6H12O6 30 Example 1 Mass-Mass Problem Glucose burns in oxygen to form CO2 and H2O according to this equation: C6H12O6 + 6 O2 6 CO2 + 6 H2O How many grams of CO2 are produced from burning 45.0 g of glucose? 1. 2. Make sure that the equations is balanced Divide the mass of the given by its molar 3. Multiply by the mole ratio 45.0 g C6H12O6 180.0 g mol-1 C6H12O6 x 6 mol CO2 1 mol C6H12O6 = 1.5 moles CO2 31 Example 1 Mass-Mass Problem Glucose burns in oxygen to form CO2 and H2O according to this equation: C6H12O6 + 6 O2 6 CO2 + 6 H2O How many grams of CO2 are produced from burning 45 g of glucose? 1.Make sure that the equations is balanced 2.Divide the mass of the given by its molar 3.Multiply by the mole ratio 4.Multiply by the molar mass of the required 45.0 g C6H12O6 180.0 g mol-1 C6H12O6 6 mol CO2 x 1 mol C6H12O6 44.0 g mol-1 CO2 x = 66.0 g of CO2 32 Example 2 Mass-Mass Problem What mass of Barium chloride is required to react with 48.6 grams of sodium phosphate according to the following reaction: 2 Na3PO4 + 3BaCl2 Ba3(PO4)2 + 6 NaCl 33 Example 2 What mass of Barium chloride is required to react with 48.6 grams of sodium phosphate according to the following reaction 2 Na3PO4 + 3BaCl2 Ba3(PO4)2 + 6 NaCl Molar Masses: Na3PO4 = 3(23.0)+31.0+4(16.0) =164 g mol-1 BaCl2 = 137.3 +2(35.5) = 208.3 g mol-1 48.6g Na3PO4 164.0 g mol-1 Na3PO4 3 mol BaCl2 x 2 mol Na3PO4 = 92.6 g of BaCl2 208.3 g mol-1 BaCl2 x 34 Example 3 What mass of carbon dioxide is produced from burning 100 grams of ethanol in oxygen according to the following reaction : C2H5OH + 3 O2 2 CO2 + 3 H2O 35 Example 3 What mass of carbon dioxide is produced from burning 100 grams of ethanol in oxygen according to the following reaction : C2H5OH + 3 O2 2 CO2 + 3 H2O Molar Masses: 100.0 g C2H5OH 46.0 g mol-1 C2H5OH = 2(12) +6(1)+ 16 = 46 CO2 = 12 + 2(16) = 44.0 x 2 mol CO2 1 mol C2H5OH X 44.0 g mol-1 CO2 = 191.3 g CO2 36 Mass to Volume Problems 37 Mass to Volume Problems • Goal: To calculate the volume of a gas that appears in a chemical reaction from the mass of a given substance in the same reaction. • The given substance is the substance whose mass is known. • The required substance is the gas whose volume is to be determined. • Remember 1 mole of any gas at STP is equal to 22.4 dm3. STP is defined as 0 oC and 1 atmosphere of pressure. 38 Steps in a Mass to Volume Problem 1. Find the gram formula masses for the given substance. 2. Convert the given mass to moles by dividing it by the molar mass 3. Multiple the given moles by the mole ratio to get the moles of the required substance 4. Multiple the number of moles of the required substance by the molar volume, 22.4 dm3 mol-1, to get the volume of the required substance. 5. This procedure is only valid if the required substance is a gas. It does not work for solids, liquids, or solutions. 39 Example 1 Mass-Volume Problem • Sucrose burns in oxygen to form CO2 and H2O according to this equation: C12H22O11 + 12 O2 12 CO2 + 11 H2O What volume of CO2 measured at STP is produced from burning 100 g of sucrose? 40 Example 1 Mass-Volume Problem Sucrose burns in oxygen to form CO2 and H2O according to this equation: C12H22O11 + 12 O2 12 CO2 + 11 H2O What volume of CO2 measured at STP is produced from burning 100 g of sucrose? 1. Find the molar mass of the given substance Molar mass of C12H22O11 = 12 (12.0) +22 (1.0) + 11 (16.0) = 342.0 g mol-1 41 Example 1: Mass-Volume Problem Sucrose burns in oxygen to form CO2 and H2O according to this equation: C12H22O11 + 12 O2 12 CO2 + 11 H2O What volume of CO2 measured at STP is produced from burning 100 g of sucrose? 2. Find moles of the given: 100 g C12H22O11 342 g mol-1 C12H22O11 = 0.292 moles 42 Example 1: Mass-Volume Problem Sucrose burns in oxygen to form CO2 and H2O according to this equation: C12H22O11 + 12 O2 12 CO2 + 11 H2O What volume of CO2 measured at STP is produced from burning 100 g of sucrose? 3. Multiply by the mole ratio: 100.0 g C12H22O11 342.0 g mol-1 C12H22O11 x 12 moles CO2 1 mole C12H22O11 = 3.51 moles CO2 43 Example 1: Mass-Volume Problem Sucrose burns in oxygen to form CO2 and H2O according to this equation: C12H22O11 + 12 O2 12 CO2 + 11 H2O What volume of CO2 measured at STP is produced from burning 100 g of sucrose? 4. Multiply by the molar volume, 22.4 dm3 mol-1. 100.0 g C12H22O11 342.0 g mol-1 C12H22O11 x 12 moles CO2 1 moles C12H22O11 x 22.4 dm3 mol-1 CO2 =78.6 dm3 44 Example 2 Mass-Volume Problem What volume of carbon dioxide gas would be produced by reacting 25.0 g of sodium carbonate with hydrochloric acid according to the following reaction: Na2CO3 + 2 HCl 2 NaCl + CO2 + H2O 45 Example 2 Mass-Volume Problem • What volume of carbon dioxide gas would be produced by reacting 25.0 g of Sodium carbonate with hydrochloric acid according to the following reaction: Na2CO3 + 2 HCl 2 NaCl + CO2 + H2O Molar Mass: Na2CO3 =2(23.0)+ 12.0 +3(16.0) =106.0 25.0 g Na2CO3 106.0 g mol-1 Na2CO3 1 mole CO2 x 1 moles Na2CO3 22.4 dm3 mol-1 CO2 x = 5.28 dm3 of CO2 46 Summary of Stoichiometric Relationships 47 Solutions and Stoichiometry • Many times the reactants and/or products of chemical reactions are water solutions. • In these cases the concentration of the solution must be determined in order to determine amounts of reactants or products • The concentration of a solution is a measure of the amount of solute that is dissolved in a given amount of solution 48 Molarity • The most common concentration unit is Molarity Molarity (M) = Moles of solute dm3 of solution 49 Molarity Calculations How many grams of NaOH are required to prepare 250 cm3 of 0.500 M solution? – Molar Mass of NaOH = 23+16+1 = 40.0 g/mol – 250 cm3 = 0.250 dm3 (0.500 mol) x ( dm3 )x (40.0 g) ( mol ) x (0.250 dm3 ) = 5.00 g 50 Molarity Calculations Calculate the concentration of a NaCl solution that contains 24.5 g of NaCl in 250 cm3 of solution. – Molar mass of NaCl = 23.0 + 35.5 = 58.5 (24.5 g NaCl) (58.5 g mol-1 ) X 1 = 1.67 M (0.250 dm3 ) 51 Stoichiometry Calculations Involving Solutions 1 Copper metal reacts with nitric acid according to the following reaction: 8 HNO3 (aq) + 3 Cu 3 Cu(NO3)2 (aq) + 4 H2O (l) + 2 NO (g) What volume of 8.00 M HNO3 would be required to consume a copper penny whose mass is 3.08 grams? (3.08 g Cu ) (8 mol HNO3) (1 dm3) ( 1000 cm3) (63.55 g mol-1 Cu ) (3 mol Cu) (8 mol HNO3) (1 dm3) = 16.2 cm3 52 Stoichiometry Calculations Involving Solutions 2 15.0 cm3 of a 0.500 M AgNO3 solution is required to precipitate the sodium chloride in 10 cm3 of a salt solution. What is the concentration of the solution? AgNO3 (aq) + NaCl (aq) AgCl (s) +KNO3 (aq) • Molar Mass NaCl = 23.0 + 35.5 = 58.5 g/mol 0.500 mol 0.0150 AgNO3 X dm3 x 1 mol NaCl X 58.5 g mol-1 dm3 1 mol AgNO3 NaCl = 0.439 g of NaCl 0.439 g of NaCl 58.5 g mol-1 x 1 0.0100 dm3 = 0.75 mol dm-3 or 0.75 M 53 Cookie Recipe Recipe Ingredients • 1 cube butter • 1 cup canola oil • 2 cups white sugar • 1 egg • 1 teaspoon vanilla extract • 1/2 teaspoon salt • 1 teaspoon baking soda • 4 1/2 cups all-purpose flour • 1 cup oatmeal In my cupboard I have: • 5 cubes butter • 8 cups canola oil • 8 cups white sugar • 12 eggs • 20 teaspoons vanilla extract • 1 pound salt • 40 teaspoons baking soda • 45 cups all-purpose flour • 30 cups oatmeal • 1 (12 ounce) package chocolate chips • 5 (12 ounce) packages chocolate chips • 5 pounds of dog biscuits How many cookies I can make with out going to the store? Makes 24 cookies 54 Limiting Reagent • Although we have been basing our calculations thus far on only one of the reactants in a chemical reaction, the reaction will only occur if we have all of the reactants • The mole ratio determines how much of each reactant we need for the reaction • Often we have an excess of one of the reactants. Then not all of that reactant will be used up. There will be some left over. • It is known as the excess reagent. • The other reactant will be used up and it will determine the amount of product we can form. • It is known as the limiting reagent 55 Limiting Reagent To determine which of the reagents is the limiting reagent 1. Calculate the number of moles of each reactant 2. Multiply first reactant by the appropriate mole ratio to get the number of moles of the second reactant that you need. 3. Compare the amount of the second reactant you have to the amount you need . 4. If you have more than you need it is in excess and the first reactant is the limiting reagent 5. If you have less of the second reactant than you need it becomes the limiting reagent 6. Use the number of moles of the limiting reagent to calculate the required quantity in the problem 56 Limiting Reagent Example 1 Barium chloride reacts with potassium phosphate as follows: 3 BaCl2 (aq) + 2 K3PO4(aq) 6 KCl (aq) + Ba3(PO4)2 (s) Calculate the mass of barium phosphate that could be formed when a solution containing 10.00 g of potassium phosphate is added to a solution containing 12.00 g of barium chloride. Molar mass potassium phosphate = 3(39.10) + (30.97) + 4(16.00) = 212.27 g mol-1 Molar mass barium chloride = (137.34) + 2(35.45) = 208.24 g mol-1 Molar mass barium phosphate = 3(137.34)+ 2(30.97)+(8)(16.00) = 601.96 g mol-1 Moles barium chloride = 12.00g / 208.24 g mol-1 = 0.05762 mol Moles potassium phosphate = 10.00g / 212.27 g mol-1 = 0.04711 mol The mole ratio is 3 mol BaCl2 to 2 mol K3PO4. While there are more moles of BaCl2 than K3PO4, It is not 1.5 times greater. Therefore BaCl2 is the limiting reagent and all other calculations will be based on barium chloride. (0.05762 mol BaCl2) (1mol Ba3(PO4)2) (601.96 g mol-1 Ba3(PO4)2 ) ( 3 mol BaCl2) = 11.56 g of Ba3(PO4)2 57 Percent Yield Stoichiometry allows us to calculate the amounts of reactants required or the amounts of products generated from a chemical reaction. Chemical reactions frequently do not proceed to completion. Hence the amount of product recovered is often less than would be predicted from stoichiometric calculations. In these situations it is helpful to calculate a percent yield. 58 Percent Yield The Theoretical Yield is defined as the amount of product(s) calculated using Stoichiometry calculations The Actual Yield is the amount of product that can actually be recovered when the reaction is done in a lab. The Percent Yield is calculated as follows Actual yield Theoretical yield x 100 59 Percent Yield Iron reacts with copper sulfate in a single replacement reaction as follows Fe (s) + CuSO4 (aq) ZnSO4 (aq) + Cu (s) 30.00 grams of iron metal were added to excess were added to excess copper sulfate dissolved in a water solution. 22.50 grams of copper were recovered. Calculate the theoretical yield of copper in this experiment . 1. First calculate the theoretical yield (30.00 g Fe) (1 mol Cu ) (55.85 g mol-1 Fe) (1 mol Fe ) (63.55 g mol-1 Cu) = 34.14 g Cu 2. Divide the actual yield by the theoretical yield and multiply by 100 22.50 g Cu 34.14 g Cu X 100 = 65.91% 60