Hydro carbons
advertisement

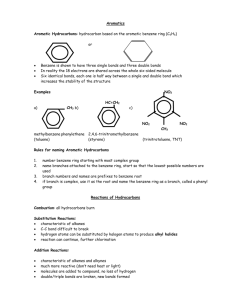
Hydro carbons Click the above picture to see the animation Hydrocarbons Hydrocarbons, in organic chemistry, family of organic compounds, composed entirely of carbon and hydrogen. They are the organic compounds of simplest composition and may be considered theoretically as the parent substances from which all other organic compounds are derived. The hydrocarbons are conveniently classified into two major groups, openchain and cyclic. In open-chain compounds containing more than one carbon atom, the carbon atoms are attached to each other to form an open chain; the chain may carry one or more side branches. In cyclic compounds the carbon atoms form one or more closed rings. The two major groups are subdivided according to chemical behaviour into saturated and unsaturated compounds. THE ALKANE SERIES GENERAL FORMULA : CnH2n The saturated open-chain hydrocarbons form a homologous series called the alkane, or paraffin, series. The composition of each of the members of the series corresponds to the formula CnH2n +2, where n is the number of carbon atoms in the molecule. Among the members of the series are methane, CH4; ethane, C2H6; propane, C3H8; and butane, C4H10. All the members of the series are unreactive; that is, they do not react readily at ordinary temperatures with such reagents as acids, alkalis, or oxidizers. The first four members of the series are gases at ordinary temperature and pressure; intermediate members are liquids; and the heavier members are semi-solids or solids. Petroleum contains a great variety of saturated hydrocarbons, and such petroleum products as petrol, heavy fuel oil, lubricating oils, petroleum jelly, and paraffin consist principally of mixtures of paraffin hydrocarbons, which range from the lighter liquid members to the solid members. THE ALKYNE SERIES The members of the alkyne series contain a triple bond between two carbon atoms in the molecule. They are very active chemically and are not found free in nature. They form a series analogous to the alkene series. The first and most important member of the series is ethyne, C2H2. CYCLIC HYDROCARBONS The simplest of the saturated cyclic hydrocarbons, or cycloalkanes, is cyclopropane, C3H6, the molecules of which are made up of three carbon atoms to each of which two hydrogen atoms are attached. Cyclopropane is somewhat more reactive than the corresponding open-chain alkane, propane, C3H8. Other cycloalkanes make up a part of ordinary petrol. Several unsaturated cyclic hydrocarbons, having the general formula C10H16, occur in certain fragrant natural oils that are distilled from plant materials. These hydrocarbons are called terpenes and include pinene (in turpentine) and limonene (in lemon and orange oils). The most important group of unsaturated cyclic hydrocarbons is the aromatics, which occur in coal tar. Although the aromatics sometimes exhibit unsaturation—that is, the addition of other substances—their principal reactions bring about the replacement, or substitution, of hydrogen atoms by other kinds of atoms or groups of atoms. The aromatic hydrocarbons include benzene, toluene, anthracene, and naphthalene Ethylene: Structure of ethylene Properties of Ethane (ethylene) •colorless gas at room temperature and pressure Melting point -169oC, Boiling point -104oC •slightly sweet smell •flammable •non-polar molecule: soluble in non-polar solvents & insoluble in polar solvents like water reactive: the active site is the double bond Readily undergoes addition reactions, for example reacts with bromine water (red-brown) to produce colorless 1,2dibromoethane CH2=CH2(g) + Br2(l) -----> CH2Br-CH2Br(g) Reactions of Ethene (ethylene) Addition of Bromine CH2=CH2 + Br2 -----> CH2BrCH2Br 1,2-dibromoethane Addition of Chlorine CH2=CH2 + Cl2 AlCl3 -----> CH2ClCH2Cl 1,2-dichloroethane Addition of Hydrogen bromide CH2=CH2 + HBr AlCl3 -----> CH3CH2Br bromoethane Addition of Hydrogen chloride CH2=CH2 + HCl AlCl3 -----> CH3CH2Cl chlorooethane Addition of Hydrogen CH2=CH2 + H2 Ni -----> 500oC CH3CH3 ethane Addition of Water CH2=CH2 + H2O H3PO4 -----> 300oC CH3CH2OH ethanol Combustion CH2=CH2 + 3O2 excess air -----> 2CO2 + 2H2O Reaction of hydro carbon •acetylene •Bert helot •carbon •catalyst •fuel cell •hydrogenation •smog •Ziegler Bertha lot : French chemist. The first professor of organic chemistry at the College de France (from 1865), he later also held high government offices, incl. that of foreign minister (1895-96). He did research in alcohols and carboxylic acids, the synthesis of hydrocarbons, and reaction rates, studied the mechanism of explosion, discovered many coal-tar derivatives, and wrote on the history of early chemistry. He was a pioneer in the use of chemical analysis as a tool of archaeology. fuel cell : Device that converts chemical energy of a fuel directly into electricity (see electrochemistry). Fuel cells are intrinsically more efficient than most other energy-conversion devices. Electrolytic chemical reactions cause electrons to be released on one electrode and flow through an external circuit to a second electrode. Whereas in batteries the electrodes are the source of the active ingredients, which are altered and depleted during the reaction, in fuel cells the gas or liquid fuel (often hydrogen, methyl alcohol, hydrazine, or a simple hydrocarbon) is supplied continuously to one electrode and oxygen or air to the other from an external source. So, as long as fuel and oxidant are supplied, the fuel cell will not run down or require recharging hydrogenation : Chemical reaction between molecular hydrogen (H2) and another element or a compound, usually in the presence of a catalyst. It may involve adding hydrogen at the sites of double or triple bonds (see bonding) to make them single bonds (i.e., to saturate an unsaturated compound; see saturation), or to aromatic compounds to make them cyclic hydrocarbons.