Influence of US Frequency on Swan Band Sonoluminescence and
advertisement

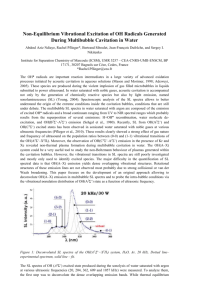
Influence of US Frequency on Swan Band Sonoluminescence and Sonochemical Activity in t-BuOH Aqueous Solutions Pflieger R.*, Ndiaye A. A., Chave T., Nikitenko S. I. Institut de Chimie Séparative de Marcoule, UMR5257, CEA-CNRS-UM2-ENSCM, Centre de Marcoule, Bat. 426, BP 17171, 30207 Bagnols-sur-Cèze, France The multibubble sonoluminescence (MBSL) spectra from organic solvents[1,2] or from aqueous solutions of organic compounds[3,4] often show emission from C2* Swan band (d3Πg → a3Πu). This band is traditionally used as a probe of intrabubble conditions. On the other hand, the C2* excited species are formed as products of a complex set of chemical reactions occurring inside the cavitation bubbles. Therefore, MBSL of C2* can be useful to study the mechanism of organic compounds sonolysis in addition to its application for cavitation thermometry. However, the relationship between sonochemical activity and MBSL has just begun to emerge. This work describes the MBSL and sonochemical studies of tBuOH sonolysis in aqueous solutions saturated with noble gases (Ar, Xe) as a function of ultrasonic frequency. The experiments have been performed at 20, 204, 362 and 613 kHz using the multifrequency sonoreactor described recently.[4] The temperature in the reactor during sonolysis was maintained at 10-12°C. Studied solutions were sparged with gas (Ar or Xe) about 30 minutes before sonication and during the ultrasonic treatment at a controlled rate of 100 mL min -1. The light emission spectra were collected in the spectral range 400-600 nm using a SP 2356i Roper Scientific spectrometer coupled to a liquid-nitrogen-cooled CCD camera with UV coating.[4] Gaseous products in the outlet gas were analyzed using a Thermo Scientific VG Prolab Benchtop quadrupole mass spectrometer. The MBSL spectra of t-BuOH aqueous solutions show emissions for the ∆υ = -1 to ∆υ = +2 vibrational sequences of C2* Swan system (Fig. 1). The ∆υ=+2 emission overlaps with CH(A-X) emission band. In general, MBSL is stronger at high-frequency ultrasound compared to 20 kHz ultrasound. However, in Xe the sonoluminescence is so bright that it can be seen by the unaided eye as a blue glow even at low ultrasonic frequency (Fig. 1d). The intensity of C2* band emission exhibits a maximum vs. t-BuOH concentration: 0.1-0.2 M at 20 kHz and (2-8)·10-3 M at high-frequency ultrasound. The huge difference in saturating concentrations of t-BuOH for low- and high-frequency ultrasound is attributed to much smaller bubble size, or in other words, to much higher surface/volume ratio for the cavitation bubbles produced at higher frequency. It was found that the relative intensities of ∆υ bands are strongly influenced by the experimental conditions. In general, the ratio of ∆υ = +1 band composed of (1-0), (2-1), (3-2), (4-3), (5-4) and (6-5) vibrational transitions and ∆υ = 0 band composed of (0-0), (1-1), (2-2), (3-3) and (4-4) vibrational transitions is higher for high ultrasonic frequency compared with that at 20 kHz, indicating higher vibrational excitation of C2* species (Fig. 1a,b). The vibrational excitation is strongly increased in the presence of Xe (Fig. 1c). Simulation of emission spectra using SPECAIR software[5] reveals Boltzmann-like behavior of C2* at 20 kHz in Ar (Tv~5800 K, Tr~4500 K), where Tv and Tr are the vibrational and rotational temperatures respectively. By contrast, in Xe at 20 kHz strong deviation from thermal equilibrium is observed (Tv~12000 K, Tr~2200 K). The vibrational temperature increases with US frequency in Ar: at 204kHz Tv~7000 K, Tr~4000 K and at 362 kHz, Tv~12000 K, Tr~6500 K. The peak area ratio R = (∆υ=+1/∆υ=0) sharply drops with an increase in t-BuOH concentration indicating quenching of higher vibrational states of C2* by organic molecules. The MBSL of C2* is in line with the spectroscopic studies of OH sonoluminescence in water revealing nonequilibrium plasma formation during acoustic collapse.[6] 2.0 v=0 v=+2 0.06 v=+1 + CH (A-X) 0.04 v=-1 0.02 0.00 400 420 440 460 480 500 520 540 SL intensity, A.U. 0.8 v=+2 + CH (A-X) 1.5 1.0 b) v=+1 v=0 0.5 0.0 400 560 , nm a) v=+2 + CH (A-X) SL intensity, A.U. SL intensity, A.U. 0.08 420 440 460 480 500 520 540 560 , nm v=+1 0.6 v=0 0.4 v=-1 0.2 0.0 400 c) d) 420 440 460 480 500 520 540 560 , nm Figure 1. MBSL from t-BuOH solutions at 20 kHz, 0.10 M t-BuOH, Ar (a), 362 kHz, 8.5·10-4 M t-BuOH, Ar (b), 20 kHz, 0.12 M t-BuOH, Xe (c), image of C2* MBSL at 20 kHz in Xe. Analysis of the gaseous products of sonolysis (H2, CH4, and C2H2) demonstrates that at higher concentration, when the MBSL is almost totally quenched, the sonochemical activity is still important, whatever the ultrasonic frequency. Even in the absence of sonoluminescence extreme conditions can still be formed inside the cavitation bubbles. References [1] Suslick K. S., Flint E. B. Nature, 330: 553-555. 1987. [2] Flint E. B., Suslick K. S. Science, 253: 1397-1399. 1991. [3] Didenko Y. T., McNamara III W. B., Suslick K. S. J. Am. Chem. Soc., 121: 5817-5818, 1999. [4] Navarro N. M., Pflieger R., Nikitenko S. I. Ultrason. Sonochem., 21 : 1026-1029, 2014. [5] Laux C. O., Spence T. G., Kruger C. H., Zare R. N. Plasma Sources Sci. Technol., 12 : 125–138, 2003. [6] (a) Ndiaye A. A., Pflieger R., Siboulet B., Nikitenko S. I. Angew. Chem. Int. Ed., 52 : 2478-2481, 2013; (b) Ndiaye A. A., Pflieger R., Siboulet B., Molina J., Dufreche J.-F., Nikitenko S. I. J. Phys. Chem. A, 116 : 4860-4867, 2012; (c) Pflieger R., Brau H.-P., Nikitenko S. I., Chem. Eur. J., 16 : 11801-11803, 2010. * E-mail: rachel.pflieger@cea.fr