Quantization of Charge, Light, and Energy
advertisement

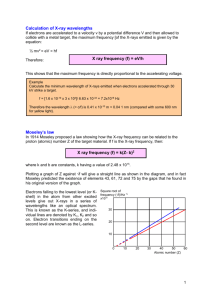
Quantization of Charge, Light, and Energy • • • • 1. Quantization of Electric Charge; 2. Blackbody Radiation; 3. The Photoelectric Effect 4. X-rays and the Compton Effect. Three great quantization discoveries: 1. Quantization of Electrical Charge 2. Quantization of Light Energy 3. Quantization of energy of Oscillating Mechanical System Quantization of Electric Charge Early measurements of e and e/m. The first estimates of the magnitude of electric charges found in atoms were obtained from Faraday law. Faraday passed a direct current through weakly conducting solutions and observed the subsequent liberation of the components of the solution on electrodes. Faraday discovered that the same quantity of electricity, F, called latter one faraday, and equal to about 96,500 C, always decomposed 1 gram-ionic weight of monovalent ions. Quantization of Electric Charge 1F (one faraday) = 96,500C 1F decomposed 1gram-ionic weight of monovalent ions. Example: If 96,500 C pass through a solution of NaCl, 23g of Na appears at the cathode and 35.5g of Cl appears at the anode. 1F =NAe - Faradays Law of Electrolysis where NA – Avogadro’s number e – minimum amount of charge, that was called an electron Discovery of electron: Thomson’s Experiment. Many studies of electrical discharges in gases were done in the late 19th century. It was found that the ions responsible for gaseous conduction carried the same charge as did those in electrolysis. J.J. Thomson in 1897 used crossed electric and magnetic fields in his famous experiment to deflect the cathode-rays. In this way he verified that cathode-rays must consist of charged particles. By measuring the deflection of these particles Thomson showed that all the particles have the same charge-to-mass ratio q/m. He also showed that particles with this charge-to-mass ratio can be obtained using any material for a source, which means that this particles, now called electrons, are a fundamental consistent of all matter. Thomson’s tube for measuring e/m. Electrons from the cathode C pass through the slits at A and B and strike a phosphorescent screen. The beam can be deflected by an electric field between plates D and E or by magnetic field. From measurements of the deflections measured on a scale on the tube’s screen, e/m can be determined. FE=qE _q - FB=qvB FE=FB qE = qvB Crossed electric and magnetic fields. When a negative particle moves to the right the particle experience a downward magnetic force FB=qvB and an upward electric force FE=qE. If these forces are balanced, the speed of the particle is related to the field strengths by v=E/B Thomson’s Experiment In his experiment Thomson adjusted E that the particles were undeflected. ┴B so This allowed him determine the speed of the particle u =E/B. He then turned off the B field and measured the deflection of the particles on the screen. Deflection of the Electron Beam. Deflection of the beam is shown with the top plate positive. Thomson used up to 200 V between the plates. A magnetic field was applied perpendicular to the plane of the diagram directed into the page to bend the beam back down to its undeflected position. With the magnetic field turned off, the beam are deflected by an amount y=y1+y2. y1 occurs while the electrons are between the plates, y2 after electrons leave the region between the plates. Lets x1 be the horizontal distance across the deflection plates. If the electron moves horizontally with speed v0 when it enters the region between the plates, the time spent between the plates is t1=x1/v0, and the vertical component of velocity when it leaves the plates is qE y qE y x1 v y a yt1 t1 m m v0 where Ey is the upward component of electric field. The deflection 2 y1 is: qE y 2 qE y x1 2 1 1 1 y1 a t t 2 y1 2 m 1 2 m v 0 The electron then travels an additional horizontal distance x2 in the field-free region from the deflection plates to the screen. Since the velocity of the electron is constant in this region, the time to reach the screen is t2=x2/v0 , and the additional vertical deflection is: y2 v y t2 qE y x1 x2 m v0 v0 The total deflection at screen is therefore qE y 2 qE y E y x12 q y y1 y2 1 2 x1 2 x1x2 2 x1x2 2 mv mv v 2 m 0 0 0 This equation cab be used to determine the charge tomass ratio q/m from measured deflection y. Example Electrons pass undeflected through the plates of Thomson’s apparatus when the electric field is 3000 V/m and there is a crossed magnetic field of 1.40 G. If the plates are 4-cm long and the ends of the plates are 30 cm from the screen, find the deflection on the screen when the magnetic field is turned off. me=9.11 x 10-31kg; q=e=-1.6 x 10-19C The Mass Spectrometer The mass spectrometer, first designed by Francis William Aston in 1919, was developed as a means of measuring the masses of isotopes. Such measurements are important in determining both the presence of isotopes and their abundance in nature. For example, natural magnesium has been found to consist of 78.7 % 24Mg; 10.1% 25Mg; and 11.2% 26Mg. These isotopes have masses in the approximate ratio 24:25:26. Schematic drawing of a mass spectrometer. Positive ions from an ion source are accelerated through a potential difference ΔV and enter a uniform magnetic field. The magnetic field is out of the plane of the page as indicated by the dots. The ions are bent into a circular arc and emerge at P2 (a plane of photographic plate or another ion detector). The radius of the circle is proportional to the mass of the ion The Mass Spectrometer In the ion source positive ions are formed by bombarding neutral atoms with X-rays or a beam of electrons. (Electrons are knock out of the atoms by the X-rays or bombarding electrons). These ions are accelerating by an electric field and enter a uniform magnetic field. If the positive ions start from rest and move through a potential difference ΔV, the ions kinetic energy when they enter the magnetic field equals their loss in potential energy, q|ΔV|: 1 2 mv q V 2 F ma 2 v qvB m R The ions move in a semicircle of radius R. The velocity of the particle is perpendicular to the magnetic field. The magnetic force provides the centripetal force v2/R in circular motion. We will use Newton’s second low to relate the radius of semicircle to the magnetic field and the speed of the particle. If the velocity of the particle is v, the magnitude of the net force is qvB, since v and B are perpendicular. mv R qB The Mass Spectrometer The speed v can be eliminate from equations 1 2 mv q V 2 mv R qB and 2 2 R q B v m2 2 2 Substituting this for v2: 1 R 2q 2 B 2 m q V 2 2 m Simplifying and solving for (m/q): 2 2 m B R q 2 V Separating Isotopes of Nickel • A 58Ni ion charge +e and mass 9.62 x 10-26kg is accelerated through a potential drop of 3 kV and deflected in a magnetic field of 0.12T. • (a) Find the radius of curvature of the orbit of the ion. • (b) Find the difference in the radii of curvature of 58Ni ions and 60Ni ions. (assume that the mass ratio is 58:60). Blackbody Radiation • One unsolved puzzle in physics in late nineteen century was the spectral distribution of so called cavity radiation, also referred to as blackbody radiation. • It was shown by Kirchhoff that the most efficient radiator of electromagnetic waves was also a most efficient absorber. A “perfect” absorber would be one that absorbed all incident radiation. • Since no light would be reflected it is called a blackbody. A small hole in the wall of the cavity approximating an ideal blackbody. Electromagnetic radiation (for example, light) entering the hole has little chance of leaving before it is completely adsorbed within the cavity. Blackbody Radiation • As the walls of the cavity absorb this incoming radiation , their temperature rises and begin to irradiate. • In 1879, the Austrian physicist J.Stefan first measured the total amount of radiation emitted by blackbody at all wavelengths and found it varied with absolute temperature. • It was latter explained through a theoretical derivation by Boltzman, so the result became known as the StefanBoltzman radiation Law: R = σT4 where R is the power radiated per unit time and per unit area of blackbody; T is the Kelvin temperature; and σ is the Stefan-Boltzman constant, σ=5.672 x 10-8 W/m2K4. Blackbody Radiation R = σT4 • Note that the power per unit area radiated by blackbody depends only on the temperature, and not of other characteristic of the object, such as its color or the material, of which it is composed. • R tells as the rate at which energy is emitted by the object. For example, doubling the absolute temperature of an object increases the energy flows out of the object by factor of 24=16. • An object at room temperature (300 K) will double the rate at which it radiates energy as a result of temperature increase of only 570. Radiation emitted by the object at temperature T that passed through the slit is dispersed according to its wavelength. The prism shown would be an appropriate device for that part of the emitted radiation in the visible region. In other spectral regions other types of devices or wavelength-sensitive detectors would be used. Spectral distribution function R(λ) measured at different temperatures. The R(λ) axis is in arbitrary units for comparison only. Notice the range in λ of the visible spectrum. The Sun emits radiation very close to that of a blackbody at 5800 K. λm is indicated for the 5000-K and 6000-K curves. Blackbody Radiation The German physicist W.Wien derived a relationship for maximum wavelength and absolute temperature, known as Wien’s displacement law: λmT = constant=(2.898 x 10-3m)K Example: The wavelength at the peak of the spectral distribution for a blackbody at 4300 K is 674 nm (red). At what temperature would the peak be 420 nm (violet)? Solution: From the Wien’s law, we have λ1T1 = λ2T2 (674 x 10-9m)(4300 K) = (420 x 10-9m)(T2) T2=6900 K • This law is used to determine the surface temperatures of stars by analyzing their radiation. It can also be used to map out the variation in temperature over different regions of the surface of an object. Such a map is called thermograph. • For example thermograph can be used to detect cancer because cancerous tissue results in increased circulation which produce a slight increase in skin temperature. Radiation From the Sun. The radiation emitted by the surface of the sun emits maximum power at wavelength of about 500 nm. Assuming the sun to be a blackbody emitter, (a) what is it surface temperature? (b) Calculate λmax for a blackbody at room temperature, T=300 K. The calculation of the distribution function R(λ) involves the calculation of the energy density of electromagnetic waves in the cavity. The power radiated out of the hole is proportional to the total energy density U (energy per unit volume) of the radiation in the cavity. The proportionality constant can be shown to be c/4, where c is the speed of the light: R = (1/4)cU Similarly, the spectral distribution of the power proportional to the spectral distribution of the energy density in the cavity. • If u(λ)dλ is the fraction of the energy per unit volume in the cavity in the range dλ, then u(λ) and R(λ) are related by R(λ)=(1/4)cu(λ) The energy density distribution function u(λ) can be calculated from classical physics. We can find the number of modes of oscillation of the electromagnetic field in the cavity with wavelength λ in the interval dλ and multiply it by average energy per mode. The result is that the number of modes of oscillation per unit volume, n(λ), is independent of the shape of cavity and is given by: n(λ) = 8πcλ-4 Rayleigh-Jeans Equation The number of modes of oscillation per unit volume: n(λ) = 8πcλ-4 According to the classical kinetic theory, the average energy per mode of oscillation is kT, the same as for a one-dimensional harmonic oscillator, where k is the Boltzman constant. Classical theory thus predicts for the energy density spectral distribution function u(λ) = kTn(λ) = 8πckTλ-4 Rayleigh-Jeans Equation u(λ) = kTn(λ) = 8πckTλ-4 This prediction, initially derived by Rayleigh, is called the Rayleigh-Jeans Law. At very long wavelength the Rayleigh-Jeans law agrees with experimentally determined spectral distribution, but at short wavelength this law predicts that u(λ) becomes large, approaching infinity as λ→0, whereas experiment shows that the distribution actually approaches zero as λ→0. This enormous disagreement between experimental measurements and classical theory for short wavelength was called the ultraviolet catastrophe. Comparison of the Rayleigh-Jeans Law with experimental data at T=1600 K. The u(λ) axis is linear. In 1900 the German physicist Max Plank by making some unusual assumptions derived a function u(λ) that agreed with experimental data. Classically, the electromagnetic waves in the cavity are produced by accelerated electric charges in the walls vibrating like simple harmonic oscillators. The average energy for simple harmonic oscillator is calculated classically from MaxwellBoltzman distribution function: f(E) = Ae-E/kT where A is a constant and f(E) is the fraction of oscillators with energy E. Maxwell-Boltzman distribution function f(E) = Ae-E/kT The average energy is then found, as is any weighted average: E Ef E dE EAe E kT dE Plank found that he could derive his empirical function assuming the energy of oscillators, and hence the radiation that they emitted, was a discrete variable that could take only the values o, ε, 2ε, 3ε,…..,nε where n is an integer, and that ε is proportional to the frequency of the oscillators, and, thus, the radiation. Plank therefore wrote the energy as En=nε=nhf n=0,1,2,….. where h is a constant now called Plank constant. The Maxwell-Boltzman distribution than becomes: fn= Ae-E/kT = Ae-nε/kT where A is determined by normalization condition that the sum of all fractions fn must be equal 1. The normalization condition f n A e n kT 1 The average energy of oscillation is then given by discretesum equivalent E En f n En Ae E kT hf x kT kT To solve this equation let put f n A e nx A(1 y y y ....) 1 2 where y=e-x. 3 x x 2 x 3 A[e e (e ) (e ) ....] 0 This sum is a series expansion of (1-y)-1, so (1-y)-1=1+y+y2+y3+… , then Σfn=A(1-y)-1=1 gives A=1-y and E En Ae 0 E kT A nhfe nhf kT Ahf ne nx 0 0 d e nx . But dx nx 1 e ( 1 y ) Note that ne nx so we have d d dy nx nx 1 2 2 ne e ( 1 y ) ( 1 y ) y ( 1 y ) dx dx dx since dy d x (e ) e x y dx dx Plank’s Law Multiplying this sum by hf and using A=(1-y), the average energy is: E hfA ne nx x hfy hfe hf (1 y) y(1 y) x 1 y 1 e 2 Multiplying the numerator and the denominator by ex and substituting for x, we obtain: E hf hf kT e 1 hc hf kT e 1 Plank’s Law Multiplying this result by the number of oscillators per unit volume in the interval dλ given by n(λ)=8πcλ-4 (the number of modes of oscillation per unit volume) we obtain the energy distribution function for the radiation in cavity: 8hc u ( ) hc kT e 1 2 5 This function is called Plank’s Law. Plank’s Law The value of Plank’s constant h can be determined by fitting the function 8hc u ( ) hc e kT 1 2 5 to the experimental data, although the direct measurement is better, but more difficult. The presently accepted value of Plank’ constant is: h = 6.626 x 10-34 J·s = 4.136 x 10-15 eV·s u ( ) 8ckT 4 Comparison of Plank’s Law and the Rayleigh-Jeans Law with experimental data at T=1600 K. The u(λ) axis is linear. Plank’s Law A dramatic example of an application of Planck’s law is the test of the predictions of the so-called Big Bang theory of the formation and expansion of the universe. Current cosmological theory suggests that the universe originated in an extremely high-temperature explosion, one consequence of which is to fill the infant universe with radiation that can be approximate with black body spectral distribution. In 1965, Arno Penzias and Robert Wilson discovered radiation of wavelength 7.35 cm reaching the Earth with same intensity from all directions in space. It was recognized soon as a remnant of the Big Bang (relict radiation). The energy density spectral distribution of the cosmic microwave background radiation. The solid line is Plank’s Law with T=2.735 K. The measurements were made by the Cosmic Back Ground Exploder (COBE) satellite. Problem 1. Thermal Radiation from the Human Body. The temperature of the skin is approximately 35°C. What is the wavelength at which the peak occurs in the radiation emitted from the skin? Problem 2. The Quantized Oscillator. A 2-kg mass is attached to a massless spring of force constant k=25N/m. The spring is stretched 0.4m from its equilibrium position and released. (a) Find the total energy and frequency of oscillation according to classical calculations. (b) Assume that the energy is quantized and find the quantum number, n, for the system. (c) How much energy would be carried away in one-quantum change? Problem 3. The Energy of a “Yellow” Photon. What is the energy carried by a quantum of light whose frequency equals 6 x 1014 Hz yellow light? What is the wavelength of this light? The Photoelectric Effect It is one of the ironies in the history of the science that in the same famous experiment of Heinrich Hertz in1887 in which he produced and detected electromagnetic waves, thus confirmed Maxwell’s wave theory of light, he also discovered the photoelectric effect led directly to particle description of light. It was found that negative charged particles were emitted from a clean surface when exposed to light. P.Lenard in 1900 detected them in a magnetic field and found that they had a charge-to-mass ratio of the same magnitude as that measured by Thompson for cathode rays: the particles being emitted were electrons. Schematic diagram of the apparatus used by P.Lenard to demonstrate the photoelectric effect and to show that the particles emitted in the process were electrons. Light from the source L strikes the cathode C. Photoelectrons going through the hole in anode A are recorded by the electrometer connected to α. A magnetic field, indicated by the circular pole piece, could deflect the particles to an electrometer connected to β, enabling the establishment of the sign of their charge and their q/m ratio. If some of emitted electrons that reaches an anode A pass through the small hole, a current results in the external electrometer circuit connected to α. The number of emitted electrons, reaching the anode, can be increased by making the anode positive with respect to cathode. Letting V be the potential difference between A and C the next picture shows the current versus V for two values of the intensity of light incident on the cathode: Photocurrent i versus anode voltage V for light of frequency f with two intensities I1 and I2 , where I2>I1 . At sufficiently large V all emitted electrons reach the anode and the current reaches its maximum value. From experiment it was observed that the maximum current is proportional to the light intensity. An expected result – if the intensity of incident light doubled the number of emitted electrons should also double. If intensity of incident light is too low to provide electrons with energy necessary to escape from the metal, no emission of electron should be observed. However, there was no minimum intensity below which the current was absent. When V is negative, the electrons are repelled from the anode and only electrons with initial kinetic energy mv2/2 grater than eV can reach the anode. From the graph we can see if the voltage is less, than –V0 no electrons reach anode. The potential V0 is called the stopping potential. It related to the maximum kinetic energy as (mv2/2)max = eV0 The experimental result, that V0 is independent of the incident light intensity was surprising – increasing the rate of energy falling on the cathode does not increase the maximum kinetic energy of the emitted electrons. In 1905, Einstein offered an explanation of this result: he assumed, that energy quantization used by Plank in the blackbody problem was a universal characteristic of light. Light energy consist of discrete quanta of energy hf. When one of this quanta, called photon, penetrates the surface of cathode, all of its energy may be given completely to electron. If Φ is the energy necessary to remove an electron from the surface (Φ is called the work function and is a characteristic of the metal), the maximum kinetic energy of the electrons leaving the surface will be (hf – Φ) and the stopping potential V0 should be given by mv2 eV0 hf 2 max This equation is referred as the photoelectric effect equation. As can be seen from mv2 eV0 hf 2 max the slope of the line on the graph V0 versus f should equal h/e. The minimum, or threshold, frequency for photoelectric effect, ft and the corresponding threshold wavelength λt are related to work function Φ by setting V0 = 0 : hft hc t Photons of frequency lower than ft ( and therefore having wavelength grater than λt ) do not have enough energy to eject an electron from the metal. For constant I Einstein’s explanation of the photoelectric effect indicates that the magnitude of the stopping voltage should be grater for f2 than f1, as observed, and that there should be a threshold frequency ft below which no photoelectrons were seen, also in agreement with experiment. Millikan’s data for stopping potential versus frequency for the photoelectric effect. The data falls on a straight line of slope h/e, as predicted by Einstein. The intercept of the stopping potential axis is –Ф/e. Example: Photoelectric Effect in Potassium The threshold wavelength of potassium is 558 nm. What is the work function for potassium? What is the stopping potential when light of wavelength 400 nm is used? Example: Photoelectric Effect in Potassium The threshold wavelength of potassium is 558 nm. What is the work function for potassium? What is the stopping potential when light of wavelength 400 nm is used? • Solution: 1 2 eV0 mv hf 2 max hf V0 e e hf t hc e e et hc 1240eV nm 2.22eV t 558nm Example: Photoelectric Effect in Potassium • The threshold wavelength of potassium is 558 nm. What is the work function for potassium? What is the stopping potential when light of wavelength 400 nm is used? 1240eV nm V0 2.22eV 400nm 3.10eV 2.22eV 0.88V hc Photoelectric Effect for Sodium A sodium surface is illuminated with light of wavelength 3x10-7m. The work function for sodium is 2.28 eV. Find (a) the kinetic energy of the ejected photoelectrons and (b) the cutoff wavelength for sodium. X Rays and the Compton Effect Future evidence of the correctness of the photon concept was given by Arthur Compton, who measured the scattering of x-rays by free electrons. The German physicist Wilhelm Röentgen discovered x-rays in 1895 when he was working with a cathode-ray tube. He found, that “rays”, originating from the point where the cathode rays (electrons) hit the glass tube, or a target within the tube, could pass through the materials opaque to light and activate a fluorescent screen or photographic film. He found that all materials were transparent to this rays to some degree, depending of the density of this materials. The slight diffraction of an x-ray beam after passing slit of a few thousands of a mm wide indicated their wavelength in other of 10-10m = 0.1nm. (a) (b) (a) Early x-ray tube and (b) typical of the mid-twenties century x-ray tube design. Diagram of the components of a modern x-ray tube. Design technology has advanced enormously, enabling very high operating voltages, beam currents, and x-ray intensities, but the essential elements of the tubes remain unchanged. X-Ray’s Diffraction Experiment soon confirmed that x-rays are a form of electromagnetic radiation with wavelength of about 0.01nm to 0.10 nm. It was also known that atoms in crystals are arranged in regular arrays that are spaced by about same distances. In 1912, Laue suggested that since the wavelength of x-rays were on the same order of magnitude as the spacing of atoms in a crystal, the regular array of atoms in crystal might act as a three-dimensional grating for diffraction of x-rays. X-Ray’s Diffraction • W.L.Bragg, in 1912, proposed a simple and and convenient way of analyzing the diffraction of xrays due to scattering from various sets of parallel planes of atoms, now called Bragg planes. • Two sets of Bragg planes are illustrated for NaCl, which has a simple crystal structure called face-centered cubic. A face-centered cubic crystal of NaCl showing two sets of Bragg planes. • Waves scattered at equal angels from atoms in two different planes will be in phase (constructive interference) if the difference in path length is an integer number of wavelength. This condition is satisfied if 2d sin θ = mλ where m = an integer This equation called the Bragg condition. Bragg scattering from two successive planes. The waves from the two atoms shown have a path difference of 2dSinθ. They will be in phase if the Bragg condition 2dSinθ = mλ is met. Schematic sketch of the Laue experiment. The crystal acts as a three-dimensional grating, which diffracts the x-ray beam and produce a regular array of spots, called a Laue pattern, on a photographic plate. Modern Laue-type x-ray diffraction pattern using a niobium diboride crystal and 20-kV molybdenum x-rays. [General Electric Company.] Incident X-ray Beam Scattered X-rays Schematic diagram of Bragg crystal spectrometer. A collimated x-ray beam is incident on a crystal and scattered into an ionization chamber. The crystal and ionization chamber can be rotated to keep the angles of incidence and scattering equal as both are varied. By measuring the ionization in the chamber as a function of angle, the spectrum of the x-rays can be determined using the Bragg condition 2dSinθ = mλ, where d is the separation of the Bragg planes in the crystal. If the wavelength λ is known, the spacing d can be determined. Two typical x-ray spectra are produced by accelerating electrons through two voltages V and bombarding a tungsten target. I(λ) is the intensity emitted with the wavelength interval dλ at each value of λ. The spectrum consist of a series of sharp lines, called the characteristic spectrum. The line spectrum is a characteristic of target material and varies from element to element. The continuous spectrum has a sharp cutoff wavelength λm which is independent of the target material but depends on the energy of the bombarding electrons. If the voltage of the x-ray tube is V in volts, the cutoff wavelength was found empirically by 1.24 103 m nm V It was pointed out by Einstein that x-ray production by electron bombardment was an inverse photoelectric effect and equation mv2 eV0 hf 2 max should be applied. The λm simply correspond to a photon with the maximum energy of the electrons, i.e. the photon emitted when the electron losses all of its kinetic energy in a simple collision. • Since the kinetic energy of the electron in the x-ray tube is 20,000 eV or larger, the work function Φ is negligible by comparison and equation mv2 eV0 hf 2 max becomes eV hf or hc hc 1240eV nm 1.24 103 nm eV eV Thus, the x-ray spectrum can be explained by Plank’s quantum hypothesis and λm can be used to determine h/e. Compton Effect Schematic sketch of Compton apparatus. X-rays from the tube strike the carbon bloke R and are scattered into a Bragg-type crystal spectrometer. In this diagram, the scattering angle is 300. The beam was defined by slits S1 and S2. Although the entire spectrum is being scattered by R, the spectrometer scanned the region around Kα line of molybdenum. Derivation of Compton’s Equation Let λ1 and λ2 be the wavelengths of the incident and scattered x rays, respectively. The corresponding momentum are E1 hf1 h P1 c c 1 and E2 h P2 c 2 using fλ = c. Since Compton used the Kα line of molybdenum ( λ = 0.0711 nm), the energy of the incident x ray (17.4 keV) is much greater than the binding energy of the valence electrons in the carbon scattering block (about 11 eV); therefore, the carbon electron can be considered to be free. Conservation of momentum gives p1 p2 pe 2 2 2 pe p1 p2 2 p1 p2 p12 p 22 2 p1 p2 cos where pe is the momentum of the electron after the collision and θ is the scattering angle for the photon, measured as shown in Figure. The energy of the electron before the collision is simply its rest energy E0 = mc2. Diagram for Derivation of Compton’s Equation After the collision, the energy of the electron is (E02 + pe2c2)1/2. Conservation of energy gives p1c + E0 = p2c + (E02 + pe2c2)1/2 Transposing the term p2c and squaring we obtain E02+c2(p1-p2)2+2cE0(p1-p2)=E02+pe2c2 or pe2=p12+p22-2p1p2+2Eo(p1-p2)/c pe2=p12+p22-2p1p2+2Eo(p1-p2)/c If we eliminate pe2 from the previous equations, we obtain E0(p1-p2)/c = p1p2(1-cosθ) Multiplying each term by hc/p1p2E0 and using λ=h/p, we obtain Compton’s equation: λ2 – λ1 = hc(1-cosθ)/E0 = hc(1-cos θ)/mc2 or λ2 – λ1 = h(1-cos θ)/mc Compton Wavelength hc 2 1 (1 cos ) me c The increase in wavelengths is independent of the wavelength λ1 of the incident photon. The quantity h has dimensions of length and is called the me c Compton Wavelength. Its value is h hc 1240eV nm 12 C 2 . 43 10 m 2.43 pm 2 5 me c me c 5.1110 eV Compton Wavelength Because λ2–λ1 is small it is difficult to observe unless λ1 is so small that the fractional change (λ2–λ1)/λ1 is appreciable. Compton used X-rays of wavelength 71.1pm. The energy of a photon of this wavelength is 1240eV nm E 17.1keV 0.0711nm hc Since this is mush greater than the binding energy of the valence electrons in most atoms, these electrons can be considered to be essentially free. Compton’s measurements confirmed the correctness of the photon concept. Reflection from Calcite If the spacing between certain planes in crystal of calcite is 0.314nm, find the grazing angles at which first and third order interference will occur for x-rays of wavelength 0.070nm. Compton Scattering at 450 X-rays wavelength λ0=0.200 000 nm are scattered from a block of material. The scattered x-rays are observed at an angle of 450 to the incident beam. Calculate the wavelength of the xrays scattered at this angle. The Minimum X-ray Wavelength Calculate the minimum x-rays wavelength produced when electrons are accelerated through a potential difference of 100 000V, a not-uncommon voltage for an x-ray tube. Photoelectric Effect in Lithium Light of wavelength of 400nm is incident upon lithium (Φ = 2.9eV). Calculate (a) the photon energy and (b) the stopping potential V0. What frequency of light is needed to produce electrons of kinetic energy 3eV from illumination from Li?