Text Questions
advertisement

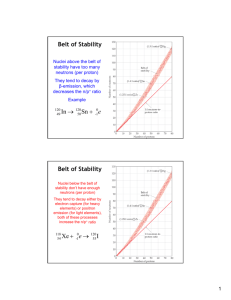
21TQ: Nuclear Chemistry Name: _________________________ Text Questions from Brown, et. al. 1. Nuclear reactions involve changes in what? 2. Why must you have an understanding of nuclear reactions and the uses of radioactive substances? 21.1 3. What are nucleons? 4. The nuclear properties of an atom depend on what? 5. What is the difference between radionuclides and radioisotopes? 6. Emission of radiation is one of the ways in which… 7. In balancing nuclear equations, what are two things that must be equal on both sides of the equation? 8. Why are we NOT concerned with the chemical form of an atom when writing nuclear equations? 9. What ARE beta particles, and how are they represented? 10. Beta emission is equivalent to what? Also, write the equation that shows this. 11. Are there electrons in the nucleus? 12. What two things DOESN’T gamma radiation change about a nucleus that emits it? 13. Why does gamma radiation usually accompany other radioactive emission? 14. What are positrons, and how are they represented? 15. What is electron capture? 16. Both electron capture and positron emission have what effect? 21.2 17. What is the so-called “nuclear force”? 18. Why is there an ever-greater need for neutrons as the number of protons in the nucleus increases? 19. Describe the belt of stability. 20. A. Above the belt of stability, nuclei have too _____ neutrons, and tend to decay by _____ emission. B. Below the belt of stability, nuclei have _____ neutrons, and tend to decay by… C. Beyond the belt of stability are the _______ nuclei that tend to decay by _______ emission. 21. What is a radioactive series? Also, how many series are there, and what nuclei does each end with? 22. What are the “magic” numbers of protons? Of neutrons? 23. How are nucleons described in the shell model of the nucleus? 24. Analogous to the pairs of electrons in molecules, evidence also suggests that… 21.3 25. What are nuclear transmutations? 26. For a particle accelerator, what is the relationship between the magnitude of the charge on the particles and their speed? 27. Why do neutrons NOT need to be accelerated to cause nuclear reactions? 28. Which elements are the transuranium elements, and why are they named thus? 21.4 29. Which radioisotopes do we NOT find in nature? 30. What type of kinetic process is radioactive decay? 31. What is the half-life? 32. List three external conditions that do NOT affect the half-life of a radioactive substance. 33. What must we do to render radioactive atoms harmless? 34. What is radiometric dating? 35. What is C-14’s half-life, and why is C-14 dating inapplicable for objects older than about 50,000 years? 36. Approximately how old do scientists believe the Earth to be? 37. What is the first-order rate constant k called? 38. Write the equation into which the first-order rate law can be transformed. 39. For the equation you wrote for Q38, what two ratios can be substituted for Nt/No? 21.5 40. Why do people who work with radioactive substances carry film badges? 41. For a Geiger counter, how are the ions and electrons produced, and what do they do? 42. Why can radioisotopes (i.e., radiotracers) be used to follow an element through its chemical reactions? 43. Where does the O2 produced in photosynthesis come from, and where DOESN’T it? 44. Why is the use of radioisotopes (i.e., radiotracers) possible? 21.6 45. In nuclear reactions, why are even small changes in mass accompanied by large changes in energy? 46. Why is it possible to treat chemical reactions as though mass is conserved? 47. How do the mass and energy changes in nuclear reactions compare to those in chemical reactions? 48. All spontaneous nuclear reactions are… 49. What did scientists discover in the 1930s? 50. What is the mass defect? 51. __________ must be added to a nucleus to break it into separated nucleons; such an addition must be accompanied by a proportional increase in ______. 52. What is the nuclear binding energy? 53. What is the relationship between the binding energy (per nucleon) and the nuclear stability? 54. Heavy nuclei gain stability through the process of ________, which is used to generate energy in _________ _______ _______. __________ amounts of energy are released when light nuclei undergo the process of _________. 21.7 55. When struck by a _____-_________ neutron, three nuclei that undergo fission are… 56. What is the difference between the critical mass and the supercritical mass? 57. What is the functional difference between control rods and the moderator? 58. A nuclear and a fossil fuel power plant are the same in what way? 59. Why have some organizations proposed that nuclear power become a major energy source? 21.8 60. What is the composition of the Sun? 61. For what two reasons is fusion appealing as an energy source? 62. What is the main problem with using fusion to generate energy? 21.9 63. Radiation can cause what two things when it is absorbed by matter? 64. Why are gamma rays particularly harmful outside the body?