PITT Restriction Enzyme/Electrophoresis Lab Protocol

Restriction Analysis of Plasmid DNA
SESSION 1/DAY 1:
RESTRICTION DIGEST
REACTIONS
*MOLECULAR BIOLOGY FINAL
*Begin Here After Biotech PP and
Electrophoresis activities
11
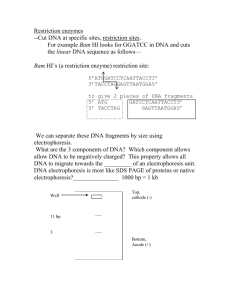
Each restriction enzyme cuts DNA wherever its recognition site appears.
Each restriction enzyme recognizes a particular sequence of nucleotides, called its restriction site.
Many recognition sites are palindromes.
BamHI
…NNN GGATCC NNN…
…NNN CCTAGG NNN…
…NNN
…NNN
G
CCTAG
HindIII
…NNN AAGCTT NNN…
…NNN TTCGAA NNN…
…NNN
…NNN
A
TTCGA
GATCC NNN…
G NNN…
AGCTT NNN…
A NNN…
12
Before We Begin: This is a restriction
Enzyme Map
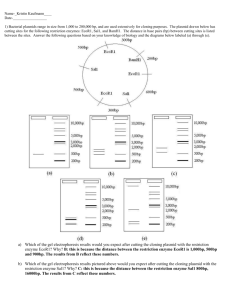
The circles below represent bacterial plasmids (loops of DNA found inside prokaryotes). The orange section is a gene for the resistance of an antibiotic (either ampicillin or kanamycin)
13
A restriction map identifies where restriction sites appear along the DNA plasmid
BamHI cuts here
HindIII cuts here
What will be different between the DNA fragments produced by cutting pAMP vs. pKAN with BamHI & HindIII?
14
The restriction enzymes and the location where they will cut on this particular plasmid is indicated on the map (i.e.
1120 means BamH1 will cut at the 1,120 th base pair starting at “12:00”)
15
Cutting with Restriction Enzymes:
If you are cutting with BamH1 For Example : The number 1120 represents the # of base pairs where BamH1 will cut from12:00 noon. So… If you are also cutting with HinDIII and you want to know the size of the piece you are cutting out take 1904bp – 1120bp =
784bp (size of what will be cut out). 4539bp - 784bp = 3755bp is size of remaining plasmid after piece cut out.
3755 bp
784 bp
16
DNAs can be distinguished from each other by restriction mapping.
3755 bp
2332 bp
1904 – 1120 = 784
4539 – 784 = 3755
784 bp
1875 bp
17
The Sample you will get for this lab will be
EITHER plasmid DNA pAMP or pKAN.
Name of plasmid
18
pAMP; Let’s get acquainted, shall we?
•
4539 base pairs •a single occurrence of the sequence
5' AAGCTT 3'
•a single replication origin
3' TTCGAA 5'
•a gene (ampr)conferring resistance to the antibiotic ampicillin (a relative of penicillin) that is cut by the restriction enzyme
HindIII
•a single occurrence of the sequence
Treatment of pAMP with a mixture of
BamHI and HindIII produces:
5' GGATCC 3'
3' CCTAGG 5'
•a fragment of 3755 base pairs carrying both the ampr gene and the replication origin that is cut by the restriction enzyme
BamHI
•a fragment of 784 base pairs
•both fragments have sticky ends
19
pKAN
•
4207 base pairs
•a single replication origin
Treatment of pKAN with a mixture of BamHI and
HindIII produces:
•a gene (kanr) conferring resistance to the antibiotic kanamycin.
•a single site cut by BamHI
•a fragment of 2332 base pairs
•a fragment of 1875 base pairs with the kanr gene
(but no origin of replication)
•a single site cut by HindIII
•both fragments have sticky ends
20
21
LAB TIME!- Using Restriction Enzymes!!!
Glove Up!
Put on a pair of lab gloves
S, M, L, XL available
Most hands will fit in M or L gloves.
Try those sizes first unless you have particularly small or large hands.
Made of nitrile (no latex = no allergies)
25
Label a Restriction Digest Tube
From the jar with the white screw cap, remove one 1.5ml microtube.
With a lab marker, label the lid of the microtube with your period number and the first initials of each team member(save room to record a number)
LID
P1
TDH
26
This is our Goal which we will complete one step at a time:
Prepare the Restriction Digest Reactions
Reaction Component
Your Plasmid DNA Sample (0.1µg/µl)
H
2
O
5X Restriction Buffer
BamHI + HindIII Restriction Enzyme mix
Total Volume
Volume to Add
5µl
9µl
4µl
2µl
20µl
27
Add Plasmid DNA
Your team was given a sample of either pAMP or pKAN plasmid DNA in a tube labeled “DNA” and a number. BE
SURE TO RECORD THIS NUMBER on your restriction digest tube lid!
From this tube, use your micropipette to measure 5μl of plasmid DNA and transfer it to your Restriction Digest tube.
At 0.1μg/μl, this 5μl contains
0.5μg or 500ng of DNA.
DNA
1…12
5μl
P1
TDH
#3
28
Add Water
From the tube labeled H
2
O, measure 9μl of
water and transfer it to your Restriction Digest tube.
H
2
O 9μl
P1
TDH
#3
29
Add Restriction Reaction
Buffer/Loading Dye
Enzymes require a chemical environment of the right pH and concentration of ions. The 5X restriction buffer is a concentrated mix that provides the environment needed for the restriction enzymes to work properly.
From the tube labeled 5X RE Buffer, measure
4μl of 5x Restriction Digest Buffer and transfer it to your Restriction Digest tube.
5X
RE
Buffer
4μl
P1
TDH
#3
30
Add Restriction Enzymes
You will cut your plasmid DNA with two restriction enzymes: BamHI and
HindIII.
From the tube labeled BamHI + HindIII measure 2μl of the BamHI and HindIII mix and transfer it to your
Restriction Digest tube.
BamHI
+
HindIII
2μl
P1
TDH
#3
31
Incubate the Restriction
Digest Reaction
Close the cap on your Restriction Digest tube and place it in the heating block set at 37°C.
The restriction enzymes work best at 37°C. The reactions will incubate for one hour, then be stored in a freezer until you examine them using gel electrophoresis.
32
SESSION 2/ DAY 2:
GEL ELECTROPHORESIS
33
Prepare Your Samples for Loading
• Do not have to add (was added to the buffer)
• Add 4µl of the 6X Loading Dye to your restriction digest sample.
If your liquids are sticking separately to the side of the tube, flick the tube with your finger and tap the bottom gently on your lab bench, or spin briefly in microcentrifuge to collect entire sample at bottom of tube.
34
Load Your Sample On The FlashGel
When called, bring to the FlashGel:
Your DNA sample
Micropipette with tip
Load 6μl of your sample into a well.
35
Write your team initials or team number below the well into which you loaded your sample.
Lane
1
Lane
2
Lane
3
Lane
4
Lane
5
Lane
6
Lane
7
Lane
8
Lane
9
Lane
10
Lane
11
Lane
12
Lane
13
1kb ladder
36
Run the Gel
A power supply provides current to the electrodes and through the buffer and gel.
The progress of migration through the gel is monitored with tracking dyes that are visible without the transilluminator.
1.2% Flash Gel
200 V
8 minutes
37
ANALYSIS OF GEL RESULTS
38
Restriction Mapping Can Be Used To
Identify Unknown DNAs
3755 bp 2332 bp
784 bp
1875 bp
39
Restriction
Fragment
Sizes pAMP:
3755,
784 pKAN:
2332
1875
1 2 3 4 5 6 7 8 9 10 11 12 13
Promega
BenchTop
1kb Ladder
1.2% 200V 8min
40
What Questions Do YOU Have?
41