Drug Metabolism
advertisement

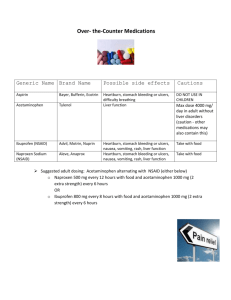
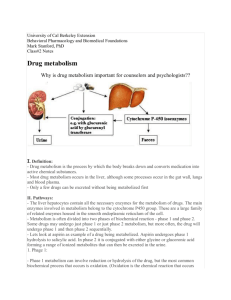
Drug Metabolism S.P. Markey Laboratory of Neurotoxicology NIMH, NIH Nov. 14, 2002 Evolution of Drug Metabolism As a Science Post WWII Pioneers • R.T. Williams – Great Britain – 1942, worked on the metabolism on TNT with regard to toxicity in munitions workers; due to the war he assembled teams to work on metabolism of sulfonamides, benzene, aniline, acetanilide, phenacetin, and stilbesterol – Developed concept of Phase 1 & Phase 2 Reactions. • Biotransformation involves metabolic oxygenation, reduction, or hydrolysis; result in changes in biological activity (increased or decreased) • Second phase, conjugation, in almost all cases resulted in detoxication. Evolution of Drug Metabolism As a Science Post WWII Pioneers • B.B. Brodie, U.S. – NYU and Laboratory of Industrial Hygiene, NYC 1949 – Metabolic fate of acetanilide and phenacetin in man (with J. Axelrod) – 1950s, NIH – pioneering studies on all aspects of drug metabolism; esp. reserpine, serotonin;hexobarbital tolerance – 1952 – R.T. Williams spent 6 months at NIH; subsequently many students went between both labs (Dick Adamson, Jim Gillette, and Sidney Udenfriend) – 1950s, Brodie lab developed the spectrophotofluorimeter (R. Bowman) Drug Metabolism Extrahepatic microsomal enzymes (oxidation, conjugation) Hepatic microsomal enzymes (oxidation, conjugation) Hepatic non-microsomal enzymes (acetylation, sulfation,GSH, alcohol/aldehyde dehydrogenase, hydrolysis, ox/red) Liver Microsomal System •Oxidative Reactions: Cytochrome P450 mediated • Examples – Formation of an inactive polar metabolite • Phenobarbital – Formation of an active metabolite • By Design: Purine & pyrimidine chemotherapy prodrugs • Inadvertent: terfenadine – fexofenadine – Formation of a toxic metabolite • Acetaminophen – NAPQI Drug NADP+ CYP eR-Ase PC CYP Fe+3 Drug Drug OH NADPH CO CYP-Fe+2 Drug CO hu CYP Fe+3 Drug OH CYP Fe+2 Drug eO2 O2 CYP Fe+2 Drug H2O 2H+ Electron flow in microsomal drug oxidizing system Cytochrome P450 Isoforms (CYPs) - An Overview • NADPH + H+ + O2 + Drug NADP+ + H2O + Oxidized Drug • Carbon monoxide binds to the reduced Fe(II) heme and absorbs at 450 nm (origin of enzyme family name) • CYP monooxygenase enzyme family is major catalyst of drug and endogenous compound oxidations in liver, kidney, G.I. tract, skin, lungs • Oxidative reactions require the CYP heme protein, the reductase, NADPH, phosphatidylcholine and molecular oxygen • CYPs are in smooth endoplasmic reticulum in close association with NADPH-CYP reductase in 10/1 ratio • The reductase serves as the electron source for the oxidative reaction cycle CYP Families • Twelve CYP gene families have been identified in humans, and the categories are based upon protein sequence homology • Most of the drug metabolizing enzymes are in CYP 1, 2, & 3 families . • CYPs have molecular weights of 45-60 kDa. • Frequently, two or more enzymes can catalyze the same type of oxidation, indicating redundant and broad substrate specificity. • CYP3A4 is very common to the metabolism of many drugs; its presence in the GI tract is responsible for poor oral availabilty of many drugs CYP Nomenclature • Families - CYP plus arabic numeral (>40% homology of amino acid sequence, eg. CYP1) • Subfamily - 40-55% homology of amino acid sequence; eg. CYP1A • Subfamily - additional arabic numeral when more than 1 subfamily has been identified; eg. CYP1A2 • Italics indicate gene (CYP1A2); regular font for enzyme CYP Tables • Human CYPs - variability and importance in drug metabolism • Isoforms in metabolism of clinically important drugs • Factors that influence CYP activity • Drugs that inhibit CYPs • Non-Nitrogenous CYP inhibitors • Extrahepatic CYPs Human Liver Drug CYPs CYP enzyme 1A2 1B1 2A6 2B6 2C 2D6 2E1 2F1 2J2 3A4 4A, 4B Level (%total) ~ 13 <1 ~4 <1 ~18 Up to 2.5 Up to 7 Extent of variability ~40-fold Up to 28 ~20-fold ~30 - 100-fold ~50-fold 25-100-fold >1000-fold ~20-fold 2E S. Rendic & F.J. DiCarlo, Drug Metab Rev 29:413-80, 1997 Factors Influencing Activity and Level of CYP Enzymes Nutrition 1A1;1A2;2E1; 3A3; 3A4,5 Smoking 1A1;1A2 Alcohol 2E1 1A1,1A2; 2A6; 2B6; 2C; Drugs 2D6; 3A3, 3A4,5 1A1,1A2; 2A6; 1B; 2E1; Environment 3A3, 3A4,5 Genetic 1A; 2A6; 2C9,19; 2D6; Polymorphism 2E1 Red indicates enzymes important in drug metabolism S. Rendic & F. J. Di Carlo Drug Metab Rev 29: 413-580, 1997 Participation of the CYP Enzymes in Metabolism of Some Clinically Important Drugs CYP Enzyme 1B1 2F1 4A 1A1 2A6 Participation in Drug metabolism (%) ~1.3 2.5 2.5 2B6 3.4 2E1 4.1 1A2 8.2 Examples of Substrates 17-Estradiol Ipomeanol Prostaglandins R-Warfarin Cyclophosphamide, Halothane Zidovudine, AZT Cyclophosphamide, Testosterone Acetaminophen, Chlorzoxazone Dapsone. Halothane Acetaminophen, Caffeine Phenacetin, (R) –Warfarin S. Rendic & F.J. Di Carlo, Drug Metab Rev 29:413-580, 1997 Participation of the CYP Enzymes in Metabolism of Some Clinically Important Drugs (cont’d) CYP Enzyme Participation in Drug Metabolism(%) 2C8,9 15.8 2C18, 19 8.3 2D6 18.8 3A4,5 34.1 Examples of Substrates Tolbutamide, Diclofenac (S) –Warfarin, Phenytoin Hexobarbital Diazepam, Omeprazole (S) –Mephenytoin Codeine, Debrisoquine Dextromethorphan “Ecstasy”, Bufuralol, Sparteine Carbamazepine, Cortisol Dapsone, Diazepam Erythromycin, Midazolam Nifedipine, Omeprazole Testosterone S. Rendic & F.J. Di Carlo, Drug Metab Rev 29:413-580, 1997 Drugs that Inhibit Drug Metabolism by Forming Complexes with CYPs Amphetamine Cimetidine Dapsone 2,5-Dimethoxy-4methylamphetamine Diphenylhydramine Erythromycin Fenfluramine Itraconazole Ketoconazole Methadone Methamphetamine Nortriptyline SKF 525A Sulfanilamide Modified from: A. Alvares and W.B. Pratt, Pathways of Drug Metabolism in Principles of Drug Metabolism (Eds. W.B. Pratt, P.Taylor) 3rd Edition, 1990 Non-nitrogenous Substances that Effect Drug Metabolism by Forming Complexes with CYPs • Grapefruit juice - CYP 3A4 inhibitor; highly variable effects; unknown constituents – D.G. Bailey, et al.; Br J Clin Pharmacol 1998, 46:101-110 • Isosafrole, safrole - CYP1A1, CYP1A2 inhibitor; found in root beer, perfume • Piperonyl butoxide & alcohol -CYP1A1, CYP1A2 inducer; insecticide constituent Overheard Conversation • At a B&B breakfast table, after grapefruit juice was served, someone remarked “A friend read the package insert with her prescription and the fine print warned against drinking grapefruit juice…is this true? Should it be avoided with all medications? How about grapefruit itself? How about orange juice?” Effect of Grapefruit Juice on Felodipine Plasma Concentration 5mg tablet with juice without Cl H CH 3 O 2 C CH 3 N H Cl CO 2 CH 3 CH 3 Cl 3A4 Cl CO 2 CH 3 CH 3 O 2 C CH 3 N CH 3 Review- D.G. Bailey, et al.; Br J Clin Pharmacol 1998, 46:101-110 Grapefruit Juice Facts • GJ or G (not OJ) elevates plasma peak drug concentration, not elimination t1/2 • GJ reduced metabolite/parent drug AUC ratio • GJ caused 62% reduction in small bowel enterocyte 3A4 and 3A5 protein; liver not as markedly effected (i.v. pharmacokinetics unchanged) • GJ effects last ~4 h, require new enzyme synthesis • Effect cumulative (up to 5x Cmax) and highly variable among individuals depending upon 3A4 small bowel basal levels Human Drug Metabolizing CYPs Located in Extrahepatic Tissues CYP Enzyme 1A1 1B1 2A6 2B6 2C 2D6 Tissue Lung, kidney, GI tract, skin, placenta, others Skin, kidney, prostate, mammary,others Lung, nasal membrane, others GI tract, lung GI tract (small intestine mucosa) larynx, lung GI tract S. Rendic & F.J. DiCarlo, Drug Metab Rev 29:413-80, 1997 Human Drug Metabolizing CYPs Located in Extrahepatic Tissues (cont’d) CYP Enzyme 2E1 2F1 2J2 3A 4B1 4A11 Tissue Lung, placenta, others Lung, placenta Heart GI tract, lung, placenta, fetus, uterus, kidney Lung, placenta Kidney S. Rendic & F.J. DiCarlo, Drug Metab Rev 29:413-80, 1997 CYP Biotransformations • Chemically diverse small molecules are converted, generally to more polar compounds • Reactions include: – – – – – Aliphatic hydroxylation, aromatic hydroxylation Dealkylation (N-,O-, S-) N-oxidation, S-oxidation Deamination Dehalogenation Aliphatic hydroxylation R CH2CH3 OH R CHCH3 Examples: ibuprofen, pentobarbital CO 2H CO 2H HO ibuprofen O O HN HN O N O O N H H pentobarbital O OH Aromatic Hydroxylation R nonenzymatic R or OH OH R O R O DNA, Pr ote in toxic reactions OH R unstable arene epoxide intermediate OH HYL1 epoxide hydrolase R OH Examples: acetanilide, phenytoin, propranolol Endogenous substrates: steroid hormones (not aromatic amino acids) phenytoin N N N N HYL1 N CYP2C8,9 N O O HO OH 3,4-dihydrodihydroxyphentoin H O O H phenytoin N N N N O HO para-hydroxyphenytoin O OH meta-hydroxyphenytoin Arene epoxide intermediate produces multiple products propranolol H H N O N O OH OH OH H N O OH OH N (or O, S)-Dealkylation R N CH3 -1e - + R N CH2 CH2 R R N CH2 CH2 CH2 R O2 CH3 -H+ CH2 CH2 R R N OH CH2 CH2 CH2 R R N H CH2 CH2 R + HCHO N-demethylation generates formaldehyde ethylmorphine N CH3 N H + O O OH ethylmorphine O O HCHO OH desmethyl-ethylmorphine N-demethylation favored over O-deakylation propranolol H H N O N O OH OH OH H N O H N O OH H OH OH 6-methylthiopurine S CH3 N N N N SH N N N N + HCHO N-Oxidation R NHOH R NH2 R N+ R _ R O R N R R Examples: chlorpheniramine, trimethylamine S-Oxidation R R S R2 Examples: chlorpromazine, cimetidine R2 S O chlorpheniramine Cl Cl N N O N N chlorpromazine O S S N Cl N N Cl N Deamination R CHCH 3 NH2 OH R C CH3 NH2 O R C CH 3 Examples: amphetamine, diazepam + NH 3 amphetamine NH2 O + NH3 Dehalogenation R1 R2 R3 C X R1 R2 R3 C. + Cl RH - R1 R2 R3 CH + . R Example: carbon tetrachloride, others include. halothane, methoxyflurane CCl 4 CHCl 3 + . R (lipid peroxidation) Non-CYP Drug Biotransformations • Oxidations • Hydrolyses • Conjugation (Phase 2 Rxs) – Major Conjugation Reactions • Glucuronidation (high capacity) • Sulfation (low capacity) • Acetylation (variable capacity) • Examples:Procainamide, Isoniazid – Other Conjugation Reactions: O-Methylation, SMethylation, Amino Acid Conjugation (glycine, taurine, glutathione) – Many conjugation enzymes exhibit polymorphism Non-CYP drug oxidations • Monoamine Oxidase (MAO), Diamine Oxidase (DAO) - MAO (mitochondrial) oxidatively deaminates endogenous substrates including neurotransmitters (dopamine, serotonin, norepinephrine, epinephrine); drugs designed to inhibit MAO used to effect balance of CNS neurotransmitters (L-DOPA); MPTP converted to toxin MPP+ through MAO-B. DAO substrates include histamine and polyamines. • Alcohol & Aldehyde Dehydrogenase - non-specific enzymes found in soluble fraction of liver; ethanol metabolism • Xanthine Oxidase - converts hypoxanthine to xanthine, and then to uric acid. Drug substrates include theophylline, 6mercaptopurine. Allopurinol is substrate and inhibitor of xanthine oxidase; delays metabolism of other substrates; effective for treatment of gout. Non-CYP drug oxidations • Flavin Monooxygenases – Family of enzymes that catalyze oxygenation of nitrogen, phosphorus, sulfur – particularly facile formation of N-oxides – Different FMO isoforms have been isolated from liver, lung (D. Ziegler, 1993, Ann Rev Pharmacol Toxicol 33:179-199) – Complete structures defined (Review: J. Cashman, 1995, Chem Res Toxicol 8:165-181) – Require molecular oxygen, NADPH, flavin adenosine dinucleotide (FAD) – Single point (loose) enzyme-substrate contact with reactive hydroperoxyflavin monoxoygenating agent – FMOs are heat labile and metal-free, unlike CYPs – Factors affecting FMOs (diet, drugs, sex) not as highly studied as CYPs FMO Oxidations H H N CH 3 N HN N H H N N N C N cimetidine + N CH 3 N O - nicotine-N-oxide nicotine S FMO3 FMO3 S HN N O H H N N N C N cimeditine S-oxide Hydrolysis Reactions Esters O O R1 O R2 R1 OH + R2 OH Example: aspirin (others include procaine, clofibrate) CO 2 H OCOCH 3 CO 2 H OH Hydrolysis Reactions Amides O R1 O NH R2 + R1 R2 NH2 OH Example:lidocaine; others include peptide drugs O N OH N H N O + NH2 Conjugation Reactions Glucuronidation CO2H O OH HO O OH O P O P O CH2 OH O OH ON O NH O UDP- -D-glucuronic acid + ROH or R 3N UGT CO2H OO R OH OH OH O-glucuronide CO2H R +R ONR OH OH OH N+-glucuronide Liver has several soluble UDP-Gluc-transferases HO 3 N O N CH3 6 O CH3 N N HO Morphine Amitriptyline Cotinine Glucuronic acid conjugation to phenols, 3°-amines, aromatic amines Conjugation Reactions Sulfation O R O S OH O R OH NH2 + N N N N H H HO O H OH O OH O P O S O O (PAPS, 3’-phosphoadenosine5’-phosphosulfate) H OH Examples: ethanol, p-hydroxyacetanilide, 3-hydroxycoumarin H2N N N H2N O N O N N N HO S O NH 2 Minoxidil O NH Minoxidil-sulfate Sulfation may produce active metabolite Conjugation Reactions Acetylation O Ar NH 2 O CoA S R NH 2 R OH R SH Ar N H + Acetyl transferase O R O CH3 CH3 O O R N H CH3 R S CH3 Examples: Procainamide, isoniazid, sulfanilimide, histamine NAT enzyme is found in many tissues, including liver Procainamide O Unchanged in Urine, 59% H2 N N H 24% Fast 17% Slow H N Unchanged in Urine, 85% N 3% O N H O N 1% NAPA 0.3% H N O O N H H N O H2 N N H H N Procainamide O H2 N N N H trace metabolite HO H N O N N H non-enzymatic O O N N H N Lupus? Drug Conjugation Example: Isoniazid - N-acetyltransferase • First line drug in the treatment of TB; normally given at a does of 5 mg/kg, max. 300 mg/day for period of 9 months • Rapid and slow acetylators first seen in TB patients; t1/2 for fast is 70 min; t1/2 for slow is 180 min • N-acetyltransferase (NAT2 isoform) is in liver, gut • Peripheral neuropathy (about 2% patients; higher doses produce effects in 10-20%) seen in slow acetylators (reversible with pyridoxine) • Hepatotoxicity also seen, esp. in older patients N N NAT2 O H H N N H H O Isoniazid CH 3 N N H O N-Acetylisoniazid N minor O OH CH3 CYP1A2 OH NH NH 2 N+ NAT2 C O O NH Carcinogenic DNA Adduct Reactive Nitrenium ion N-Acetylation may trigger nitrenium ion formation Additional Effects on Drug Metabolism • Species Differences – Major differences in different species have been recognized for many years (R.T. Williams). • Phenylbutazone half-life is 3 h in rabbit, ~6 h in rat, guinea pig, and dog and 3 days in humans. • Induction – Two major categories of CYP inducers • Phenobarbital is prototype of one group - enhances metabolism of wide variety of substrates by causing proliferation of SER and CYP in liver cells. • Polycylic aromatic hydrocarbons are second type of inducer (ex: benzo[a]pyrene). – Induction appears to be environmental adaptive response of organism – Orphan Nuclear Receptors (PXR, CAR) are regulators of drug metabolizing gene expression PXR and CAR Protect Against Xenobiotics target genes CAR xenobiotics RXR xenoprotection PXR cytoplasm nucleus S.A. Kliewer CYP3A Inducers Activate Human, Rabbit, and Rat PXR rifampicin PCN Cell-based reporter assay dexamethasone RU486 clotrimazole troglitazone tamoxifen 1 3 5 7 9 11 13 15 17 19 Reporter activity (fold) S.A. Kliewer CYP3A Regulation • Expressed in liver and intestine • Activated by xenobiotics • Bind to Xenobiotic Response Elements xenobiotics rifampicin PCN dexamethasone RU486 clotrimazole troglitazone tamoxifen ? XRE xenobiotics (e.g., drugs) endobiotics (e.g., steroids) CYP3A liver intestine CYP3A HO-xenobiotics HO-endobiotics • Protect against xenobiotics • Cause drug-drug interactions S.A. Kliewer et al. Endo Rev 23:687, 2002 Pregnane X Receptor (PXR) human PXR DNA Ligand rabbit PXR 94% 82% mouse PXR 96% 77% rat PXR 96% 76% • PXR is one of Nuclear Receptor (NR) family of ligand-activated transcription factors. • Named on basis of activation by natural and synthetic C21 steroids (pregnanes), including pregnenolone 16-carbonitrile (PCN) • Cloned due to homology with other nuclear receptors • Highly active in liver and intestine • Binds as heterodimer with retinoic acid receptor (RXR) S.A. Kliewer Constitutive Androstane Receptor (CAR) • • • • • CAR CAR CAR DNA Ligand PXR PXR PXR 66% 41% Highly expressed in liver and intestine Binds response elements as RXR heterodimer High basal transcriptional activity without ligand Sequestered in cytoplasm Activated by xenobiotics – phenobarbital, TCPOBOP (1,4-bis[2-(3,5dichloropyridyloxy)]benzene) S.A. Kliewer Plasticity in the PXR Binding Pocket Volume: SR12813 hyperforin 1280 Å3 1544 Å3 S.A. Kliewer PXR Structure • Large, elliptical hydrophobic cavity • The cavity changes shape to accommodate different ligands • PXR is ideally suited to function as xeno-sensor xenobiotics PXR RXR xenobiotic metabolism S.A. Kliewer PXR and CAR Regulate Overlapping Genes PCN (PXR) TCPOBOP (CAR) • Phase I enzymes Cyp3a11 Cyp2b10 Aldh1a1 Aldh1a7 (3.5x) (12x) (2.1x) (1.6x) (3.4x) (110x) (1.9x) (1.9x) (2.8x) (16x) (15x) • Phase II enzymes Liver RNA Ugt1a1 Gst-a1 • Transporters Mrp2 Mrp3 Oatp2 (3.0x) (9.2x) (2.0x) (1.9x) S.A. Kliewer Acetaminophen • Acetanilide – 1886 – accidentally discovered antipyretic; excessively toxic (methemoglobinemia); para-aminophenol and derivatives were tested. • Phenacetin introduced in 1887, and extensively used in analgesic mixtures until implicated in analgesic abuse nephropathy; 1946, Lester reported conjugated para-aminophenol as major metabolite of acetanilide • 1948-49 Brodie and Axelrod recognized acetaminophen as the major active metabolite in phenacetin • CAR modulates acetaminophen toxicity [Science (Oct 11) 298:422, 2002] Acetaminophen and p-Aminophenols HN COCH 3 HN COCH 3 NH2 Acetanilide, 1886 (accidental discovery of antipyretic activity; high toxicity) 75-80% 70-90% NH2 OC 2 H5 HN OC 2 H5 Phenacetin or acetophenetidin, 1887 (nephrotoxic, methemoglobinemia) COCH 3 Recognized as active metabolite of acetanilide and phenacetin in 1948 (Brodie &Axelrod); popular in US since 1955 OH Acetaminophen, 1893 Acetominophen Metabolism HN COCH 3 ~60% HN O COCH 3 O OH CO 2 H OH HO OH ~35% CYP2E1* CYP1A2 CYP3A4 N COCH 3 HN O COCH 3 SO 3 H *induced by ethanol, isoniazid Protein adducts, O NAPQI Oxidative stress N-acetyl-p-benzoquinone imine Toxicity Acetaminophen Toxicity •Acetaminophen overdose results in more calls to poison control centers in the United States than overdose with any other pharmacologic substance. •The American Liver Foundation reports that 35% of cases of severe liver failure are caused by acetaminophen poisoning which may require organ transplantation. •N-acetyl cysteine is an effective antidote, especially if administered within 10 h of ingestion [NEJM 319:15571562, 1988] •Addition of N-acetyl cysteine to acetaminophen tablets proposed to prevent liver toxicity. [British Medical Journal, Vol. 323, Sept. 15, 2001] Acetaminophen Protein Adducts HN COCH 3 N COCH 3 CYP2E HS-Protein O OH H2NProtein Protein S N COCH 3 HN COCH 3 HN COCH 3 S Protein O OH NH Protein OH S.D. Nelson, Drug Metab. Rev. 27: 147-177 (1995) J.L. Holtzman, Drug Metab. Rev. 27: 277-297 (1995) NAPQI toxicity linked to CAR activation, GSH depletion N COCH 3 SH HN COCH 3 glu-cys-gly GLY S CYS Glutathione S-Transferase (GST Pi) O OH GLU SH glu-cys-gly Phenobarb TCPOBOP CAR androstanol GST Pi toxicity oxidative stress mechanism ? Protective effect. Liver cells die (pale areas) when exposed to high doses of acetaminophen (left), but a CAR inhibitor protects against such damage (right). Jun Zhang,* Wendong Huang,* Steven S. Chua, Ping Wei, David D. Moore Science, October 11, 298:422, 2002 Acetaminophen toxicity mechanism • Mice nulled for glutathione S-transferase are resistant to acetaminophen toxicity – equal amounts of acetaminophen protein adducts formed in null and wild type suggesting protein adducts may not be toxic – hepatic GSH lowered in wild type (but not in KO) after acetaminophen • CAR nulled mice are also resistant to acetaminophen toxicity – hepatic GSH lowered in wild type (but not in KO) after acetaminophen – CAR-humanized mice demonstrate same toxicity response • N-acetyl cysteine is an effective agent to block GSH depletion and rescue from liver damaging toxicity • NAPQI-protein adduction or NAPQI-GSH depletion-oxidative stress....to be continued Terfenadine (Seldane©) OH HO N Terfenadine in the News • DHHS/FDA: Terfenadine; Proposal to Withdraw Approval of Two New Drug Applications – Federal Register 62, January 14, 1997 • Hoechst Marion Roussel To Promote Switch From Seldane to Allegra – Independent News Service, January 14, 1997 • Citing Its Side Effects, F.D.A. Weighs Ban on Allergy Drug – The New York Times, January 14, 1997 • FDA Wants Drug Seldane Off Market – The Washington Post, January 14, 1997 • Hoechst’s First Quarter Results Below Forecasts – Independent News Service, May 7, 1997 Terfenadine • Developed in 1980s as a 2nd generation H1antihistamine; from introduction in 1985, prescriptions > 16 million in 1991 • First generation antihistamines are lipophilic ethylamine derivatives that readily penetrate the CNS and placenta - objective of 2nd generation is minimal CNS effects (non-sedating), not crossing the blood brain barrier; longer acting • Cardiac side-effects are serious - inhibition of potassium channels by unmetabolized parent drug causes prolongation of QT interval leading to life threatening arrythmia (torsades de pointes); first recognized at USUHS in 1989 (Monahan BP et al, JAMA 1990; 264:2788-2790.) • Drugs or substances inhibiting terfenadine metabolism (grapefruit juice, ketoconazole, itraconazole, antimicrobials) or liver dysfunction exacerbate the side effects Terfenadine Metabolism OH HO N Terfenadine (Seldane) CYP3A4 OH HO N Fexofenadine (Allegra) CO 2 H Drug Metabolism - WWW Information Resources •http://www.icgeb.trieste.it/p450/ – Directory of P450 Containing Systems; comprehensive web site regarding all aspects of chemical structure (sequence and 3D) of P450 proteins from all species; steroid ligands; links to related sites including leading researchers on P450 •http://www.panvera.com/tech/dmeguide/index.html – Drug Metabolism Resource Guide - catalog with useful information and characteristics of natural and recombinant drug metabolizing enzymes; assay methods •http://www.netsci.org/Science/Special/feature06.html – Site contains essay “The emerging role of ADME in optimizing drug discovery and design” RJ Guttendorf, Parke-Davis •http://www.fda.gov/cder/guidance/ – Site contains many useful documents regarding drug metabolism and FDA recommendations including "Drug Metabolism/Drug Interaction Studies in the Drug Development Process: Studies in Vitro", FDA Guidance for Industry.