File
advertisement

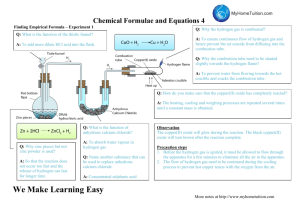
Exam 2 SI CHM 111 1. (30pt) Name the following compounds by stating the formula when given the chemical name and chemical name when given the formula: a. lithium acetate ____________________ l. B2F6 _______________________________ b. Copper (I) oxide ___________________ m. Magnesium hydroxide_______________ c. VSO3_________________________ n. nitrogen tribromide _________________ d. SO____________________________ o. sulfur hexachloride __________________ e. Aluminum sulfide___________________ p. OsCrO4__________________________________________ f. CsClO4____________________________________________ q. Br- _______________________________ g. Ammonium phosphate ________________ h. MnO2_______________________________________ i. vanadium (V) cyanide ________________ j. NH3 ___________________________________ k. PBr3 ________________________ 2. (15 pt) Calculate the molar mass of compounds (f) and (i) in problem 1 and calculate the number of molecules and grams present in 25.17 mmole of compound (f). 3. (15pt) Balance the following reactions: a. AgI + Fe2(CO3)3 FeI3 + Ag2CO3 b. c. The combustion of pentane, C5H12. S8 + O2 SO2 4. (15pt) Magnesium metal burns in oxygen to form magnesium oxide. How many grams of magnesium oxide are formed from 17 mg of magnesium? 5. (25pt) Magnesium reacts with nitric acid to produce hydrogen gas and magnesium nitrate. a. Write the balanced reaction b. If I start this reaction with 40 grams of magnesium and an excess of nitric acid, how many grams of hydrogen gas will I produce? c. If 1.7 grams of hydrogen is actually produced, what was my percent yield of hydrogen? d. How much nitric acid was consumed and how much magnesium nitrate was formed from the 40 grams of Mg?