Facilities and operations
advertisement

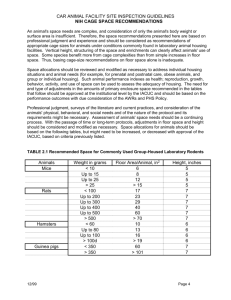
Speakers and topics Hilton J. Klein, M.S., V.M.D. – Overview and introduction Kathryn A. L. Bayne, M.S., Ph.D., D.V.M. – Review of commonly cited facility problems James F. Taylor, D.V.M., M.S. – Design of facilities - the AAALAC perspective Stephen T. Kelley, M.S., D.V.M. – Performance standards and facility design and operation Hilton J. Klein, M.S., V.M.D. Overview and introduction What is a program? Rising costs of research and research trends … Rapidly increasing R&D costs New targets from genomics Flexibility and adaptability Research trends of animal use – Dog and monkey use - USDA reports show decline – Rodent use – Institution dependent – Academic vs. industry – NIH/PHS funding increases – Overall/general animal use Animal regulations – Dog, monkey space and care - U. S.; Europe Flexibility and adaptability Future – Regulation of rats, mice, birds - space? – Operational issues – Energy – Maintenance – New technologies – Transgenics and new species – Genomics and proteomics – Other drivers for the way animals are used – Social (Cont’d) Facilities operation and design Scientific programs Laboratory animals Veterinarians Engineers Community Building considerations Research objectives New construction Renovation Flexibility and adaptability Utilities use Adjacencies Operational costs Operation and design tools (Some examples) Information sharing - network Computer aided design Computational fluid dynamics Information and management An Integrated Database for Managing Animal Study Proposals and Animal Inventory for the Small Animal Facility. T. Calzone, J. S. Montijo, M. B. St.Claire, and E. Lamoreaux. 2001. Lab Animal 30(2):28-31. A Comprehensive, Bar Coded System for the Management of Animal Information in a Research Facility. C. Pryor, D. Frankenfield, H. Klein, W. Terpeluk, S. Washington, N. T. Mourad. 2001. Lab Animal 30(2):36-38. Software for Lab Animal Facilities. G. Novak and T. Schub. 2001 Lab Animal 30(2):39-43. Conclusion: renovations or construction will require systems for information management access and retrieval for effective colony and facility management. Design and operational considerations Qualification Performance standards approach Factory acceptance testing (FAT) Dirty cage set up Microbiology tests Physical testing Installation qualification (IQ) Operational qualification (OQ) Performance standards "Performance standards define an outcome in detail and provide criteria for assessing that outcome, but do not limit the methods by which to achieve that outcome." Standards used Guide for the Care and Use of Laboratory Animals (NRC 1996) EEC 86/609 CoE Convention National legislation Reference resources (“Ag Guide,” AVMA Panel on Euthanasia, etc...) Hager Hauler Summary and conclusions As demand for animal space changes, we must design, construct, and operate facilities in a flexible and adaptable manner. The use of R&D resources is rising as new therapeutic targets are identified. Summary and conclusions (Cont’d) Animal research resources are coupled to R&D and we must determine strategies to address operational issues through facility design and automation-performance standards. Team approaches are highly effective for scientists, administration, engineers, lab animal to address and solve space and operational issues. Summary and conclusions Certain future areas in lab animal facilities opportune for change include: – – – – Room design and layout Facility design and layout New technological advances Automation (cont’d) Kathryn A. L. Bayne, M.S., Ph.D., D.V.M. Review of commonly cited facility problems Over 640 accredited institutions ... … in 18 countries 10 ,0 00 25 24, 9 ,0 00 99 50 49, 9 ,0 00 99 10 -9 9, 0, 99 00 9 020 19 0, 00 9,99 09 49 9, 99 9 >5 00 ,0 00 99 9 00 09, 1, 00 0 <1 Proportion of accredited units By facility size (sq. feet) 100 90 80 70 60 50 40 30 20 10 0 Percent of Total Animal care and use program deficiencies 13% Institutional Policies 12% Laboratory Animal Medicine Veterinary Care 5% 70% Physical Plant Facilities mandatory deficiencies 1. Facility HVAC 2. Facility safety 3. Facility maintenance 4. Facility sanitation 5. Facility design 6. Facility illumination 7. Facility storage 8. Facility security The top three deficiencies IACUC function Occupational health and safety program Heating, ventilation and air conditioning system performance HVAC mandatories (Ranked in order of most common) 1. Data not available at site visit 2. Not maintaining temperature range 3. Not maintaining air changes (ventilation) 4. Not maintaining humidity range 5. Not meeting recirculated air standards 6. Animal room temperature and humidity not monitored Common HVAC findings Air exchange rate (10-15 ach) Relative humidity levels Air recirculation/filtration Air pressure differentials HVAC purposes (Guide) Supply adequate oxygen Remove thermal loads Dilute gaseous and particulate contaminants Adjust moisture content Create static-pressure differentials Space, temperature and humidity criteria Dry bulb temperature – Adjustable +/- 2° – Fixed, minimum 66°F or 68°F – Individual room or zone Space, temperature and humidity criteria Relative humidity – Adjustable or fixed, 30-70% RH – Individual room or zone HVAC purposes (NIH Ventilation Design Handbook) Balance air quality, animal comfort and energy efficiency to provide cage environments that optimize animal welfare and research efficiency. Provide a healthy and comfortable environment for researchers and animal caregivers. Factors Room size Air change rates Pressurization Type and location of diffusers Type and location of racks/cages Factors Species Bedding type Cage change frequency www.aaalac.org/connection_1su1998.htm Contains: Details on codes, regulations and standards. Laboratory animal facilities planning and design including architectural finishes and costs issues. Overview of equipment and mechanical systems. Available in CD ROM or Spiral Bound book. James F. Taylor, D.V.M., M.S. Design of facilities – the AAALAC perspective Critical elements for success Define what the facility needs to accomplish Provide flexibility to accommodate future needs Knowledgeable users and A&E/planners: • • • • Plan, program, design, and construct Define decision making matrix Consider operational and life-cycle costs Review, review, review! Constantly focus on ‘Achilles heels’ Include commissioning/validation Program needs Animal procedures - vivarium or laboratories Surgical or diagnostic radiography suites In-house diagnostic needs Need for floor drains Containment/contamination control Imaging requirements Sizing major installed equipment Impact of design on labor costs Separation of functions Animal ops from personnel areas Disease-status separation Species conflicts/incompatibilities Noise Operational adjacencies Established colonies vs. new arrivals Cage sanitation Cage storage/cage staging Procedure rooms Surgical suite and associated support spaces Loading dock and associated in/out functions Indirect adjacencies requiring accommodation Horizontal vs. vertical design Elevators Stairways Security Windows/external light Mechanical systems distribution Support columns Security Traffic flow vs. efficiency of design System of corridors Containment/contamination control Safety and security (emergency egress) Personnel entering or using facility Animal resource staff; research staff Maintenance/service staff; Visitors Access to support spaces (offices, training) Horizontal versus vertical construction Facility integrity considerations Seismic Vibration External water - vertical & horizontal Inherent insulation Acoustic control Floor loading considerations Institutional infrastructure Electrical Central steam & chilled water Water and sewage systems Communications Security Facility maintenance Interstitial space = max. flexibility Avoid maintenance devices above animal room drop ceilings Consider space/access for repair of all installed equipment! Mechanical systems Design HVAC for worst case Dedicate to animal facility Provide component redundancy Ductwork integrity (minimal leakage) Air pressure differential control needs RH control (none, zone, room-by-room) Additional exhaust needs Floor drains Drain diameter/grating critical Location – Center vs. side; trench vs. surface Obviously should be low point of room Cap drains in infrequently used rooms – Consider installed but capped as contingency Ventilation characteristics Computational fluid dynamics Air supply diffusers Exhaust grilles - number and location Room exhaust filters to protect HVAC Pressure differentials Stability of temp and RH control Floors Chemical and wear resistance Life cycle cost - maintenance burden Epoxy, seamless vinyl, MMA, terrazzo, tile Surface preparation and cure times! Provide continuous cove Installer expertise is paramount Walls Structural requirements (caging systems) Space (and renovation) costs of CMU versus RFP Noise control Life cycle cost - maintenance burden Epoxy, tile, RFP Surface preparation and cure times! Ceilings Bottom of floor above or suspended Access requirement Sanitizability Integrity – impact upon pest control program Fit and finish protection Wall guards - bumpers Door jamb guards Corner guards Interior curbs Critical dimensions Door heights and widths (net clearances) Cage wash equipment chamber (H&W) Elevator door heights Autoclave height, width and depth Corridor widths + turning radiuses at corners, elevator lobbies, etc. Corridor devices & other protuberances (signs, fire extinguishers, telephones, etc.) Doors Avoid hollow doors (pest management) Door hardware - long-term integrity is critical Hinges Door closures Door handle design Security (electric strike) Metal versus fiberglass versus wooden Electrical system Early identification of high-demand equipment Emergency (stand-by power) needs HVAC Emergency lighting Emergency egress; surgery/ICU areas Animal holding; outlets for equipment Perimeter and internal security Assure sufficient distribution, placement and number of outlets Illumination Dual light levels Fixture placement relative to rack positions to maximize cage level illumination Light-cycle automation minimizes inadvertent lighting errors Cage wash Consider automation for large facilities Consider equipment throughput capacities versus manpower costs Solid waste management - soiled bedding Ergonomics of cage wash tasks deserve priority treatment Personnel safety and comfort deserve priority consideration Assure adequate space around machines for maintenance and repair! Critical elements for success Define what the facility needs to accomplish Provide flexibility to accommodate future needs Knowledgeable users and A&E/planners – – – – Plan, program, design, and construct Define decision making matrix Consider operational and life-cycle costs Review, review, review! Constantly focus on ‘Achilles heels’ Include commissioning/validation Stephen T. Kelley, M.S., D.V.M. Performance standards and facility design and operation AAALAC International uses recognized references for performance standards… www.aaalac.org/resources Examples of references which address facility design and operation Guide for the Care and Use of Laboratory Animals, 1996, National Research Council, National Academy of Sciences. Animal Welfare Act - 9 CFR Chapter 1, Subchapter A, Animal Welfare. Biosafety in microbiological and biomedical laboratories, 4th Ed., 1999, HHS Publication No. (CDC) 93-8395. References (Continued) Occupational Health and Safety in the Care and Use of Research Animals, 1997. National Research Council, National Academy of Sciences. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, Federation of Animal Science Societies, First Revised Edition, January 1999. References (Continued) Guide to the Care and Use of Experimental Animals, Canadian Council on Animal Care. Vol. 1, 1993. Guide to the Care and Use of Experimental Animals. Canadian Council on Animal Care. Vol. 1, 1993. References (Continued) European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. Council of Europe (Convention ETS 123), 1985. Council Directive on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes. European Union (Directive 86/609/EEC), 1986. Evaluation criteria Performance vs. engineering Evaluation responsibility Institutional Animal Care and Use Committee Facility management Engineering Operational considerations Size of the program Nature of animal use – Species – Flexibility requirements Geographical location & environment Facility type and construction Facility location and traffic patterns Security Public access Signs Locks and other measures Traffic reduce potential for contamination Personnel areas Clerical / office areas Rest rooms / locker rooms Eating areas Animal species Species requirements Microbiological status Containment Support functions Surgery – Dedicated? Procedure Necropsy Cage Wash Receiving Laboratories Maintenance Identification of deficiencies Prioritization of repair Conducting repairs Documentation of the maintenance program Surfaces Walls, ceilings, floors – Frequency – Space – Materials and methods Heating ventilation and air conditioning Monitoring – – – – – Personnel Manual / automatic Temperature & humidity Air flow direction Evidence of animal abnormalities Frequency Maintenance Operational aspects Heating ventilation and air conditioning Special requirements Biosafety and fume hood maintenance and certification Necropsy Inhalant anesthetics Plumbing Monitoring – Drinking water systems – Sanitation water systems – Drains Illumination Light timers (timer overrides) Light intensity Natural light Observational conditions Noise Animal issues Personnel safety issues Operational issues Storage facilities Adequacy Appropriate for use or separation – – – – Food Bedding Clean cages Chemicals Sanitation facilities Prevent cross contamination Control aerosols - personnel protection Monitoring effectiveness Maintenance Use of vacuums Use of chemicals Standard operating procedures and training The key element necessary to assure high levels of performance standards: Well trained and dedicated personnel Case studies Case Study #1 HVAC Observation Site visitors conducted a site visit at a respected, small research institute conducting infectious disease studies involving Biosafety Level 2 agents. There were a total of six (6) animal rooms housing either rats or mice. The HVAC report below was provided as an attachment to the program description. Room No. Filtration Air Exchanges Air Pressure Differential 1101 HEPA 11.8/hr (fresh) Negative 1202 HEPA 8.2/hr (fresh) Negative 1303 HEPA 7.8/hr (fresh) Negative 1404 HEPA 10.4/hr (fresh) Negative 1505 HEPA 12.0 (fresh) Negative 1606 HEPA 8.0 (fresh) Negative Surgery/Necropsy HEPA 14.0/hr (fresh) Negative Follow up All rooms were sanitized at weekly intervals by wetmopping the floor and wiping the walls down with an appropriate mild quaternary ammonium disinfectant. Cages were sanitized appropriately twice weekly. Bedding was also changed once in a hood between cage sanitation cycles. Upon entering the rooms, site visitors observed the following cage and stocking densities … Follow up Room 1101 rats-4 plastic cages (2/box) Room 1202 rats-8 plastic cages (2/box) Room 1303 mice-15 plastic cages (3/box) Room 1404 mice-12 plastic cages (2/box) Room 1505 mice-10 plastic cages (4/box) Room 1606 rats-8 shoebox cages (2/box) Suggestion for improvement … Case Study #2 Elevator access Observation A site visit to a large university biomedical research program indicated that a small colony (n=25 adults) of macaques was housed in the top floor of a “satellite” building. The research involved behavioral testing and brain imaging which was conducted in separate laboratories within the same building. The behavioral test lab and the imaging lab were accessible only by an elevator which was also used to transport non-laboratory personnel. Cage washing facilities were located in the basement of the building. Findings The macaques were specific pathogen free and were known to be CHV-1 (Herpes “B” virus) negative by ELISA and Western Blot. Cages were covered by Tyvek® shrouds for transport to and from cage wash. Soiled cages were sprayed with povidine-iodine solution prior to transport to the cage wash area. Elevators were “locked out” to personnel when transport to and from the labs was performed and the elevators were sanitized after use. Review of documents revealed no problems. Suggestions for improvement Suggest a security review to assure the potential for escaped animals is minimized in the elevator, the behavioral testing lab, and the imaging lab. Suggest the labs be evaluated for wearing adequate PPE and whether human patients were imaged in the imaging lab, as well as any health risks to personnel and patients. Case Study #3 After-hours monitoring Upon careful review of the written Program Description, site visitors concluded that after-hours monitoring of the animal rooms in a 45 year old animal facility consisted of: a) recording the high-low temperature readings in the room on a log sheet by the animal caretaker, and b) the security guard making rounds to ensure the corridor and hallway doors are closed. This process was confirmed during the site visit. more… Case Study #3 After-hours monitoring (cont’d) Additional background information revealed a steam injector valve in the room humidification control system had stuck in the open position overnight six months prior to the site visit. This room housed 50 rats on a respiratory/inhalation study at the time. Animal care staff realized the room temperature had reached 105ºF overnight because of the steam valve defect. Fifteen animals were found dead the next morning. Within two days, the study was terminated because of twenty (80%) percent mortality in the controls and test animals. Excessive respiratory problems were observed in the remaining animals which invalidated the study. Suggestions for improvement There were no after hours monitoring mechanism for monitoring HVAC system performance in the facility and for alerting responsible personnel for malfunctions. To minimize the risk to animal health and control variables that might confound research and testing data, a process whereby appropriate personnel are notified when environmental variables fall outside Guide recommended ranges should be implemented. Suggestions for improvement AAALAC International must be notified of such events under the recent changes in the by-laws for accredited institutions. The institution was reminded of the requirement to notify OLAW as well as AAALAC.