receptor - Learning
advertisement

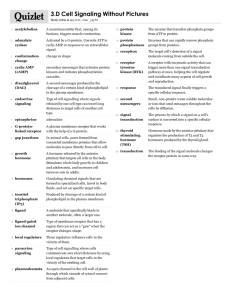
Dr. C. Behrens Dr. T. Mofokeng About 100 years ago Starling coined the term “hormone” to describe secretin, a substance secreted by the small intestine into the bloodstream to stimulate pancreatic secretion. The first connection between a viral oncogene, a mutated receptor tyrosine kinase and human cancer was made in 1984 Neural Endocrine, Neuroendocrine Paracrine Autocrine Tropic hormones Typical examples are luteinizing hormone, and folliclestimulating hormone, thyroid-stimulating hormone and adrenocorticotrophic hormone (LH, FSH, TSH, ACTH) G protein coupled receptors Tyrosine kinase receptors Serine/threonine kinase receptors Ion channels Cytokine receptors Nuclear receptors describes the affinity of the hormone for the receptor. It is usually a straight line, but several mechanisms contribute to a non-linearity: More than one type of receptor that binds to hormone More than one binding site Cooperative interactions among binding sites 3 general classes of hormones: Protein and polypeptides The steroids the amino acid tyrosine In or on the surface of the cell membrane. In the cell cytoplasm In the cell nucleus (nuclear receptor superfamily) These hormones bind to intracellular receptors that function in the nucleus of the target cell to regulate gene expression. Classical hormones that utilize intracellular receptors include thyroid and steroid hormones. Change membrane permeability Activate intracellular enzymes when they combine with their receptors Activate Genes by binding with intracellular receptors Down Regulation: binding of a hormone with its target cell receptors often causes the number of active receptors to decrease Up Regulation: The stimulating hormone induces the formation of more receptor molecules than normal by the proteinmanufacturing machinery of the target cell. Internalization: Receptor endocytosis causes internalization of cellsurface receptor Hormone-receptor complex is subsequently dissociated Receptor trafficking may then result in recycling back to the cell-surface or internalized receptor may undergo lysosomal degradation. Lead to impaired hormone signalling by down- regulation. Common structural mechanisms : an extracellular domain containing the ligand-binding site, a single transmembrane domain and an intracellular portion that includes the tyrosine kinase catalytic domain. Once activated, they are capable of phosphorylating other protein substrates. There are at least two distinct mechanisms whereby tyrosine phosphorylation regulates protein function Phosphatidylinositol 3-Kinase Grb-2 and the activation of RAS: Grb-2 and the activation of RAS: mSos is capable of activating the Ras, a small G protein that plays an important role in intracellular signalling pathways. mSos activates Ras by catalysing the exchange of GTP to GDP in the guanine nucleotide-binding site of Ras. This in turn triggers the activation of a cascade of serine/threonine-specific protein kinases including Raf, These pathways downstream from Ras contribute to theability of tyrosine kinases to promote cell growth and regulate the expression of various genes. Receptor tyrosine kinase and disease patterns: Overexpression or mutations are responsible for oncogenic RTK’s Alterations include deletions or mutation within the extracellular region or alterations of the catalytic domain result in a constitutive active RTK. Mutations within the transmembrane domain lead to ligand-independent kinase activation as reported for the RTK HER2/neu. Intervention Strategies: Possibility for the development of target – specific drugs and new anti-cancer therapies Promising approach: small molecule drugs that selectively interfere with the intrinsic tyrosine kinase activity and thereby block receptor The tyrphostins, which belong to the quinazolines: specifically inhibit the EGFR This family comprise of four members: EGFR/ErbB1, which was the first RTK to be molecularly cloned HER2/ErbB2 HER3/ErbB3 HER4/ErbB4 The EGFR is frequently overexpressed in nonsmall cell lung, bladder, cervical, ovarian, kidney and pancreatic cancer and occurs with very high incidence in squamous cell carcinomas of the head and neck. A very potent mechanism of constitutive EGFR activation in a variety of human cancers is autocrine stimulation via growth factor loops Since aberrant EGFR signalling is implicated in many cancers and seems to correlate with poor prognosis, it is an excellent target for anti-cancer therapy Currently the monoclonal antibody-based treatments using cetuximab (C225) represent the most successful anti-EGFR therapies C225 is directed against the extracellular domain of the EGFR and inhibits receptor activation and downstream signalling by blocking ligand binding to the EGFR and inducing receptor internalization. The RTK HER2 was originally identified at both the transforming gene in a chemically transformed rat neuroblastoma cell line and has been most frequently implicated in human neoplasias and cancer The role of HER2 in tumour growth: The transforming ability of HER2 is linked to cell survival Progression through the cell cycle is critically dependent on active complex formation of CDK with cyclin D1 key regulator of G1/S phase transition of the cell cycle. Overexpression of HER2 increases the metastatic potential of human potential of human breast and lung cancer cells and correlates with the number of lymph node metasteses in node-positive breast cancer patients Elevated levels of a processed HER2 extracellular domain metastatic breast cancer patients seem to reduce the efficacy of certain chemotherapy combinations consist of two extracellular α subunitsand two membrane spanning β subunits bearing the tyrosine kinase domain and the autophosphorylation site While insulin is mostly a metabolic hormone, IGF-I and IGF-II are crucial for normal development and carcinogenesis The insulin receptor closely resembles the type 1 receptor for IGFs. In the inactive form of insulin receptor kinase, TYR1162 is located in a position so that it blocks protein substrates from binding to the active site, When the three tyrosine residues in the activation loop become phosphorylated by autophosporylation of three tyrosine residues (Tyr1158, Tyr1162,Tyr1163) in the activation loop, an important conformation change occurs as a result the ability to bind both ATP and protein substrates. They are expressed on endothelial cells during embryonic development and are the key regulators for angiogensis, a process which leads to the formation of new blood vessels developing from pre-existing ones solid tumours beyond a diameter of 1-2mm requires de novo formation of a vascular network http://www.bumc.bu.edu/busm-pathology/busm-faculty-profiles/naderrahimi-phd/ The FGFs and their designated receptors play roles in normal development but also in tumour formation and progression. Suramin, a compound that complexes heparinbinding growth factors, such as FGF-2, has been shown to inhibit cell migration and FGF-2 mediated induction of urokinase-plasminogen activator. The gene responsible for MEN2 was identified as the Rearranged during transformation (RET) RTK Has intra and extracellular codons expressed during embryogenesis in the peripheral nervous system and in the development of the neural crest and the kidneys Men 2A : Autosomal dominant Men and women equally effected Medullary Thyroid carcinoma Pheochromocytoma Primary Parathyroid hyperplasia Men 2B: Autosomal dominant Medullary Thyroid carcinoma Pheochromocytoma NOT HYPERPARATHYROIDISM The tumour develops at an earlier age and is more aggressive than in MEN2A Several features in common with Receptor Tyrosine kinase: N-terminal extracellular domains, which bind ligand A single transmembrane domain C-terminal intracellular domains, which possess protein kinase activity The two classes of differ with respect to enzymatic specificity Mediate the biological actions of the transforming growth factor (TGF)-β family of ligands Cytokine receptors activate members of the Janus family of tyrosine kinases: four known JAKs (JAK1, JAK2, JAK3, TYK2). Precise regulation of the cytokine receptors is required for normal function Ligand binding to cytokine receptors normally activates JAKs rapidly and transiently. Activated JAKs and STATs are associated with cellular transformation. For example, a single acquired activating point mutation in JAK2 is present in the majority of patients with a myeloproliferative disorder. G protein-coupled receptors (GPCR) are an e evolutionarily conserved gene superfamily with members in all eukaryotes from yeast to mammals. All share a common structural feature, seven membrane-spanning helices, but various subfamilies diverge in primary amino acid sequence and in the domains that serve in ligand binding, G prot coupling and interaction with other effector proteins. All GPCR act as guanine nucleotide exchange factors G protein- regulated effectors include enzymes of second messenger metabolism such as adenyl cyclase and phospholipase effect on hormone secretion, muscle contraction and a variety of other physiologic functions. Hormones bind receptor: coupling of the receptor to a G-protein This receptor becomes the activated enzyme adenyl cyclase, Protrudes to the inside of the cell. Catalyzes the formation of cAMP, and cAMP has a multitude of effects inside the cell to control cell activity Adrenocorticotropic Hormone (ACTH) Angiotensin II (epithelial) Calcitonin Catecholamines (B receptors) Corticotropin –releasing hormone (CRH) Follicle –stimulating Hormone (FSH) Glucagon Human Chorionic Gonadotropin (HCG) Luteinizing hormone (LH) Secretin Thyroid Stimulating Hormone (TSH) Vasopressin Angiotensin II (vascular smooth muscle) Catecholamines Gonadotropin-releasing hormone Growth Hormone releasing hormone Oxytocin Thyroid releasing hormone (TRH) Vasopressin GPCRs have long been a major targer for drug development. Defects in GPCRs are an important cause of a wide variety of human diseases. Diseases caused by G protein coupled receptor loss of function mutations Receptor Disease Inheritance V2: Vasopressin Nephrogenic diabetes insipidus X-linked ACTH Familial ACTH Resistance Autosomal recessive GHRH Familial GH deficiency Autosomal recessive GnRH Hypogonadotropic Hypogonadism Autosomal recessive GPR54 Hypogonadotropic Hypogonadism Autosomal recessive FSH Autosomal recessive LH Hypergonodadotropic ovarian dysgenesis Male pseudohermaphroditism TSH Familial Hypothyroidism Autosomal recessive CA 2+ sensing Autosomal dominant Autosomal recessive Melanocortin 4 Familial Hypocalcuric hypercalcemia, Neonatal severe primary hyperparathyroidism Obesity PTH/PTHrP Blomstrand Chondrodysplasia Autosomal recessive Autosomal recessive Autosomal recessive Receptor Diseases caused by G protein coupled receptor gain-of-function mutations Disease Inheritance LH Familial male precocious puberty Autosomal dominant TSH Sporadic hyper functional thyroid modules Noninherited (somatic) TSH Familial nonautoimmune hyperthyroidism Autosomal dominant Ca2+ sensing Familial hypocalcemic hypercalcuria Autosomal dominant PTH/PTHrP Jansen’s metaphyseal chondrodysplasia Autosomal dominant V2 vasopressin Nephrogenic inappropriate Autosomal dominant antidiuresis Bind to intracellular receptors Regulate gene expression. Lipophilic: readily absorbed from GIT Excellent targets for pharmaceutical interventions. Range from those used to treat specific hormone deficiencies to those used to treat common multigenic diseases such as inflammation, cancer and type 2 Diabetes. The carboxyl (C-) terminus of the nuclear receptor is responsible for hormone binding. Major structural change: internal folding of the most C-terminal helix (H12) Class Hormone Receptor Classical Hormones Thyroid Hormone Thyroid hormone receptor Subtype α, β Estrogens Estrogens Receptor (ER) subtype α, β Testosterone Androgen receptor (AR) Progesterone Progesterone receptor (PR) Aldosterone Mineralocorticoid receptor (MR) Cortisol Glucocorticoid receptor (GR) 1,25-(OH)2-vit.D Vit.D receptor (VDR) All-trans-retinoic acid 9-cis-retinoic acid retinoid acid receptor , subtypes α, β and γ Fatty acids Peroxisome proliferators activated receptor (PPAR) , subtypes α, β and γ Oxysterols Liver X receptor (LXR) , subtypes α, β Bile acids Bile acid receptor (BAR) Vitamins Metabolic intermediates and products Xenobiotics Retinoid X receptor (RXR) , subtypes α, β and γ Pregnane X receptor (PXR) Constitutive androstane receptor (CAR) Vitamin D are synthesized and stored in the skin and activated by ultraviolet light; vit D can also be derived from dietary sources. Vit D is then converted in the liver to 25(OH) Vitamin D and in the kidney to 1-25(OH)2Vitamin D3. 1-25(OH)2-Vitamin D3 is the most potent natural ligand of vitamin D receptor (VDR). Up to date version 18.2 Steroid Hormones increase protein synthesis The sequence of events: 1. Steroid hormone enter cytoplasm 2. Bind to specific receptor protein 3. Combined receptor protein-hormone then diffuses into or is transported into nucleus 4. Bind to a specific points on the DNA strands 5. Activates the transcription process of mRNA 6. mRNA diffuses into cytoplasm 7. Translation at ribosomes to form new proteins Hormone overproduction Hormone underproduction Altered tissue responses to hormones Resistance to hormones can be causes by a variety of genetic disorders. Tumours of the endocrine glands Genetic Defects in Receptor function Mutations can lead to a decrease in the number of receptors by Impairing receptor biosynthesis Inhibiting the transport of receptors to their normal location in the plasma membrane Accelerating the rate of receptor degradation Mutations can impair the intrinsic activities of the receptor. Auto-antibodies directed against Cell-surface receptors Inhibitory anti-receptor autoantibodies were first identified in myasthenia gravis (where nicotinic acetylcholine receptor impair neuromuscular transmission, apparently by accelerating receptor degradation) Insulin resistance is caused by at least two mechanisms: The anti-receptor antibodies inhibit insulin binding to the receptor The antibodies accelerate receptor degradation Graves-disease provided the first example of stimulatory anti-receptor antibodies, where there are auto-antibodies directed against the thyroid-stimulating hormone (TSH) receptor. These anti-receptor antibodies activate the TSH receptor, thereby stimulating growth of the thyroid gland as well as hypersecretion of the hormone. IGF AKROMEGALY Pegvisomant RECEPTOR TYROSINE KINASE HER 2 BREAST CANCER Trastuzumab cAMP G-COUPLED PROTEIN RECEPTORS Secondary messenger systems SERINE KINASE TGF NEPHROGENIC SYSTEMIC FYBROSIS IN ADVANCED RENAL FAILURE NUCLEAR HORMONE VIT D OSTEOPOROSIS CYTOKINE RECEPTORS JAK POLYCYTEMIA VERA HORMONE RECEPTORS Calciumcalmodulin complex ?? Imatinib Imatinib trials busy