Reaction Profiles & Calorimetry Worksheet | Chemistry
advertisement

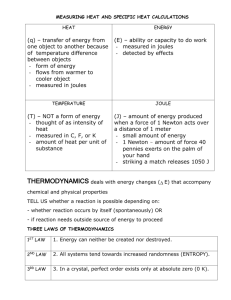
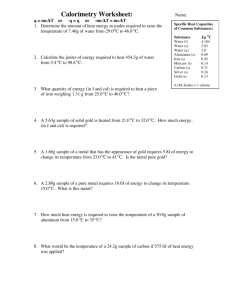
Do Now Name ……………………………………………. Team ………………………. Title: reaction profiles Date ……………………… Questions 1 and 2 refer to the following potential energy diagram: 1 The forward reaction is an exothermic / endothermic reaction. 2 The enthalpy change for the forward reaction will be negative /positive. Questions 3 and 4 refer to the following potential energy diagram: 3 The activation energy (Ea) for the reverse reaction will be kJ 4 The enthalpy change (ΔH) for the forward reaction will be kJ mol-1 5 The above reaction is Exothermic / Endothermic 6 On the graph draw a reaction profile for the same reaction if a catalyst is used Rapid Knowledge Name ……………………………………………. Team ……………………… Title: Definitions Date ……………………… Define each of the following: 1st Ionization energy Electronegativity Polar bond Polar Molecule Charles Law Precipitation Exothermic Information sheet That ΔH for a reaction can be determined in the lab by using a calorimeter. That a calorimeter measures the heat flowing into and out of a system as the reaction proceeds. Before we look at calorimeters we need to understand. Heat Capacity The heat capacity of an object is the amount of energy needed to increase the temperature of a substance 1 degree, so the units are J / oC. Heat capacity is an extensive property, so a large object has a larger heat capacity than a small object made from the same material. Heat Capacity = heat supplied / temperature rise The heat capacity of an object depends on both the quantity and types of matter in the object. Generally in order to compare heat capacties of different substances we must relate heat capacity to the amount of material in question. One way to do this is to refer to a mole of substance, the heat capacity then becomes the Molar heat capacity. This is of course mainly applicable to materials that are pure substances. A more useful procedure is to compare heat capacities for one gram of material. This is called the specific heat capacity or simply specific heat. Specific heat is the quantity of heat required to raise the temperature of one gram of material one degree Celsius (or one kelvin). We get the specific heat when we divide the heat capacity of a material by its mass. Specific heat = heat capacity / mass = C / m To find the heat q required to raise the temperature of a sample by a certain amount, you multiply the specific heat of the substance, s, by the mass in grams, m, and the change in temperature, t. q=sxmx t Suppose we wish to know the final temperature of a object after a given quantity of heat has been added to it. We can use the equation just above to calculate t which, since we have added heat will be the increase in temperature. Once we have the value of the temperature increase we can add that to the initial temperature giving us the final temperature. Had we removed heat from the object or cooled it we would have subtracted the t from the initial temperature to obtain a lower final temperature. The heat capacity of a substance is the amount of heat energy it must consume in order to raise its temperature by 1K or 1ºC It can be expressed using either the units joules or calories. A calorie is defined as the amount of heat needed to raise the temperature of 1.00 gram of water by 1.00ºC, and joules are the SI units for energy; 1 calorie = 4.184 joules. Every pure substance has a unique heat capacity, and the heat capacity of 1 mol of a pure substance is known as its molar heat capacity (J/mol-K or J/mol-ºC). The heat capacity of 1 gram of a substance is known as its specific heat (J/g-K). Students will need to know q = mCpΔT and that q = quantity of heat (joules or calories) m = mass in grams of pure substance being heated ΔT = Tf - Ti (final – initial) Cp = specific heat capacity (J/g ºC) Students need to be able to recall specific heat of liquid water is 4.184 J/g ºC (or 1.00 cal/g ºC), which is unusually high (this is due to hydrogen bonding). Example calculation How much energy would be needed to heat 450 grams of copper metal from a temperature of 25.0ºC to a temperature of 75.0ºC? The specific heat of copper at 25.0ºC is 0.385 J/g ºC. Explanation Given mass, two temperatures, and a specific heat, you have enough values to plug into the specific heat equation q = mCpΔT and plugging in your values you get q = (450 g) (0.385 J/g ºC) (50.0ºC) = 8700 J Specific heat capacity The specific heat capacity (symbol c or s, also called specific heat) of a substance is defined as heat capacity per unit mass. The SI unit for specific heat capacity is the joule per kilogram kelvin, the amount of heat energy (measured in joules) required to raise the temperature of one kilogram of the substance by one kelvin. Heat capacity can be measured by using calorimetry. Often though the units for specific heat capacity is given in joules/(gram.Kelvin) watch out for this Factors that influence heat capacity measurements Temperature: For example, measuring the heat capacity of water produces different results if the starting point is 20 °C rather than 60 °C. Intermolecular forces: If a fluid has strong intermolecular forces (such as hydrogen bonding in water) then the heat capacity is likely to be higher. Table of specific heat capacities Substance Phase Specific heat capacity J/(kg·K) Aluminium solid 900 Brass solid 377 Copper solid 385 Diamond solid 502 Ethanol liquid 2460 Gold solid 129 Graphite solid 720 Iron solid 444 Mercury liquid 139 Oil liquid ≈ 2000 liquid 4186 solid (0 °C) 2060 Water Standard ambient temperature and pressure used unless otherwise noted. To convert specific heat capacity into J/(g.K) simply divide the value in the table by 1000 Constant-Pressure Calorimetry Recall that H is defined as the quantity of heat transferred under constant pressure A calorimeter for such measurements would have the following general construction: Note that the pressure regulator could be just a vent to allow the pressure to be maintained at atmospheric pressure For reactions which involve dilute aqueous solutions, the specific heat of the solution will be approximately that of water (4.18 J g-1 K-1) The heat absorbed by an aqueous solvent is equal to the heat given off by the reaction of the solutes: qaq solvent = -qrxn Remember: If a reaction gives off heat, it is exothermic and H is negative. The enthalpy of the products is less than the enthalpy of the reactants H = Hproducts-Hreactants In our calorimeter with an aqueous solution, if the reaction of the solutes is exothermic, the solution will absorb this heat and increase in temperature. Thus, for an exothermic reaction: The solutes have a lower final enthalpy after the reaction (H negative) The solution has a higher final enthalpy after the reaction (H positive) So, to determine the actual Hrxn we would invert the sign of the Hsoln (the actual value we will measure) Here’s an example similar to the experiment we will do this week. 50 ml of 1.0 M HCl and 50 ml of NaOH are combined in a constant pressure calorimeter. The temperature of the solution is observed to rise from 21.0 oC to 27.5 oC. Calculate the enthalpy change for the reaction (assume density is 1.0 gram/ml, and that the specific heat of the solution is that of water). Tsolution = 27.5 - 21.0 oC = 6.5 oC (K) specific heat = 4.18 J g-1 K-1 mass = (100 ml)*(1 gram/ml) = 100 grams Hsolution = qp = (4.18 J g-1 K-1)*(100 g)*(6.5 K) = 2,717 J We know that Hrxn = -Hsolution therefore, Hrxn = - 2,717 J Note: exothermic reactions have negative values for Hrxn thus, if the reaction gave off heat (i.e. raised the temperature of the solution) you know that the sign for Hrxn must be negative. What is the enthalpy change on a molar basis? HCl + NaOH -> NaCl + H2O This is the balanced equation, and we combined: (0.05 liters HCl)*(1.0 mol/liter) = 0.05 moles HCl and (0.050 liters NaOH)*(1.0 mol/liter) = 0.05 moles NaOH The stoichiometry for HCl and NaOH in this reaction is 1:1, so they are combining in stoichiometrically equivalent amounts and will produce 0.05 moles of NaCl (and H2O). So, the enthalpy change for the production of 0.05 moles of NaCl in the above reaction would be: -2717 J for each 0.05 moles NaCl, or -2717 J/.05 moles = 54,340 J/mole = 54.34 kJ/mole Summary of the terms and symbols Quantity Symbol Unit ΔT o mass m Difference between the final and initial temperatures for a process temperature change C or K o Measure of the kinetic energy of molecular motiom T C or K temperature J q Energy transfer that produces or results from a difference in temperature heat heat capacity specific heat capacity Meaning g Amount of material present C Heat required to change the J oC-1 or J temperature of a substance one K-1 degree s J oC-1 g-1 Heat required to change the or J K-1 g- temperature of one gram of a 1 substance one degree Lecture Notes More explaining Diagrams Definitions quotes and smart phrases Equations Key points Lecture Notes More explaining Diagrams Definitions quotes and smart phrases Key points Equations Independent practice Name ……………………………………… Team ………………………. Title: potential energy profiles Date ……………………… Calculate the specific heat of each substance using the information given. Does it take the same amount of heat to raise 1 gram of the substance by 1 oC? Substance water Iron Mercury NaCl (s) Heat added 55.5 J 3.01 kJ 451 J 132 J Mass 2.09 g 38.9 g 111.1 g 8.89 g ΔT 6.35 oC 172 oC 29.0 oC 106 oC Specific Heat J/g oC 2. How much heat is required to raise 15.0 g of water from 20.0 oC to 50.0 oC? 3. A 1.29 g piece of lead has a temperature of 26.0 oC. If it loses 1.90 J of heat to its surroundings, what is its new temperature. Specific heat of lead is 0.128 J/g oC 4. A 55.1 g piece of metal is heated to a temperature of 45.1 oC, and placed into a cup containing 359 g of water at 20.0 oC. The final temperature of the water and metal is 22.3oC. a. How much heat energy did the water absorb? b. How much heat energy did the metal release to the water? c. What is the specific heat of the metal? 5. A 36.9 g sample of metal is heated to 100.0 oC, and then added to a calorimeter containing 141.5 g of water at 23.1 oC. The temperature of the water rises to a maximum of 25.2 oC before cooling back down. a. Did the water absorb heat or did it release heat? b. How many joules of heat was exchanged between the water and the metal? c. What is the identity of the metal? 6. You are measuring the heat of a reaction with a bomb calorimeter with a heat capacity of 2.50 J/oC. The reaction caused the temperature of the calorimeter to increase by 2.00 oC. a. Is the reaction exothermic or endothermic? b. How much heat was given off by the reaction? 7. 1.84 g of magnesium is burned in a bomb calorimeter. The temperature of the calorimeter rose from 21.30 oC to 28.56 oC. The heat capacity of the calorimeter is 6.27 kJ/oC. How many joules of heat were produced by the reaction? Exit Ticket Name …………………………………………… Team ………………………. Title: exo endo Date ……………………… 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron. 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? 3. To what temperature will a 50.0 g piece of glass raise if it absorbs 5275 joules of heat and its specific heat capacity is 0.50 J/g°C? The initial temperature of the glass is 20.0°C. 4. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6.75×104 joules of heat, and its temperature changes from 32°C to 57°C. 5. 100.0 mL of 4.0°C water is heated until its temperature is 37°C. If the specific heat of water is 4.18 J/g°C, calculate the amount of heat energy needed to cause HWK Name ………………………………………… Team ………………………. Title: Potential energy profiles Date ……………………… 1) What is the specific heat of a substance that absorbs 2.5 x 103 joules of heat when a sample of 1.0 x 104 g of the substance increases in temperature from 10.0C to 70.0C? 2) A 1.0 kg sample of metal with a specific heat of 0.50 KJ/KgC is heated to 100.0C and then placed in a 50.0 g sample of water at 20.0C. What is the final temperature of the metal and the water? 3) A 2.8 kg sample of a metal with a specific heat of 0.43KJ/KgC is heated to 100.0C then placed in a 50.0 g sample of water at 30.0C. What is the final temperature of the metal and the water? 4) Calculate the amount of heat needed to increase the temperature of 250g of water from 20oC to 56oC. 5) Calculate the specific heat capacity of copper given that 204.75 J of energy raises the temperature of 15g of copper from 25o to 60o. 6) 216 J of energy is required to raise the temperature of aluminium from 15 o to 35oC. Calculate the mass of aluminium. (Specific Heat Capacity of aluminium is 0.90 JoC-1g-1). 7) The initial temperature of 150g of ethanol was 22oC. What will be the final temperature of the ethanol if 3240 J was needed to raise the temperature of the ethanol? (Specific heat capacity of ethanol is 2.44 JoC-1g-1). Solutions to Specific Heat Problems q = (m)(t)(cp) where q is the energy in joules m is the mass t is the change in initial temperature to the final temperature cp is the specific heat (heat required to raise 1 g of the substance 1C) 1) q = (m)(t)(cp) 2.5 x 103 J = (1.0 x 104 g)(70C-10C)(cp) [2.5 x 103 J] = cp 4 [1.0 x 10 g][70C-10C] 0.0042 J = cp gC -------------------------------------------------------------------------------------------------------------2) qmetal = (m)(t)(cp) qmetal = qwater = (m)(t)(cp) qwater (m)(t)(cp) = (m)(t)(cp) [1.0Kg][100C-Tf][0.50KJ] = [50.0g][Tf-20C][4.184 J] [ KgC] [ gC ] [1.0Kg][1000g][100C-Tf][0.50KJ][ 1Kg][1000J] = [50.0g][Tf-20C][4.184 J] [ 1Kg] [ KgC][1000g][ 1KJ] [ gC ] [1000g][100C-Tf][0.50J] = [50.0g][Tf-20C][4.184 J] [ gC] [ gC ] [1000g][100C-Tf][0.50 J/gC] = [Tf-20C] [ 50.0g] [4.184 J/gC] [2.39][100C-Tf] = [Tf-20C] 239C-2.39Tf = Tf-20C 239C + 20C = Tf + 2.39Tf 259C = 3.39Tf 259C = Tf 3.39 76C = Tf -------------------------------------------------------------------------------------------------------------3) qmetal = (m)(t)(cp) qmetal = qwater = (m)(t)(cp) qwater (m)(t)(cp) = (m)(t)(cp) [2.80Kg][100C-Tf][0.43KJ] = [50.0g][Tf-30C][4.184 J] [ KgC] [ gC ] [2.8.0Kg][1000g][100C-Tf][0.43KJ][ 1Kg][1000J] = [50.0g][Tf-30C][4.184 J] [ 1Kg] [ KgC][1000g][ 1KJ] [ gC ] [2800g][100C-Tf][0.43J] = [50.0g][Tf-30C][4.184 J] [ gC] [ gC ] [2800g][100C-Tf][0.43 J/gC] = [Tf-30C] [ 50.0g] [4.184 J/gC] [24.08][100C-Tf] = [Tf-30C] 2408C-24.08Tf = Tf-30C 2408C + 30C = Tf + 24.08Tf 2438C = 25.08Tf 2438C = Tf 325.08 97C = Tf