measuring heat and specific heat calculations
advertisement

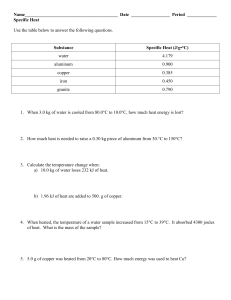
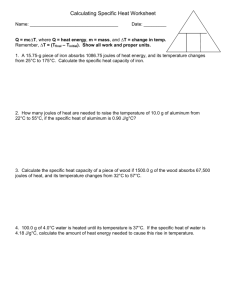
MEASURING HEAT AND SPECIFIC HEAT CALCULATIONS HEAT (q) – transfer of energy from one object to another because of temperature difference between objects - form of energy - flows from warmer to cooler object - measured in joules TEMPERATURE (T) – NOT a form of energy - thought of as intensity of heat - measured in C, F, or K - amount of heat per unit of substance ENERGY (E) – ability or capacity to do work - measured in joules - detected by effects JOULE (J) – amount of energy produced when a force of 1 Newton acts over a distance of 1 meter - small amount of energy - 1 Newton ~ amount of force 40 pennies exerts on the palm of your hand - striking a match releases 1050 J THERMODYNAMICS deals with energy changes (∆ E) that accompany chemical and physical properties TELL US whether a reaction is possible depending on: - whether reaction occurs by itself (spontaneously) OR - if reaction needs outside source of energy to proceed THREE LAWS OF THERMODYNAMICS 1ST LAW 1. Energy can neither be created nor destroyed. 2ND LAW 2. All systems tend towards increased randomness (ENTROPY). 3RD LAW 3. In a crystal, perfect order exists only at absolute zero (0 K). How do we measure HEAT? WHAT IS HEAT CAPACITY? energy required to raise temperature of substance by 1 °C (1 k) - Every pure substance has unique heat capacity - Expressed as Joules 1 Calorie = 4.184 Joule WHAT IS SPECIFIC HEAT ? (Cp) energy required to raise temperature of substance by 1 °C Specific Heat varies depending on: 1. Type of substance 2. State of matter of substance 3. Temperature of the reaction S.H. of ICE S.H. of liquid water = 2060 J/kg°C = 4180 J/kg°C How do we measure HEAT? 1. Mathematically using HEAT EQUATION q = m x Cp x ∆T q = ___________ m = __________ Cp = specific heat (J/kg°C) T = __________°C EX. How much energy would be needed to heat .450 Kg of Copper metal from a temperature of 25 °C to 75 °C? Specific Heat of Copper at 25 °C = 385 J/kg °C q = m (Cp) ∆T PLUG AND CHUG! q = q = 2.Using an instrument called Calorimeter