Hedgehog pathway (878 KB ) - Sigma
advertisement

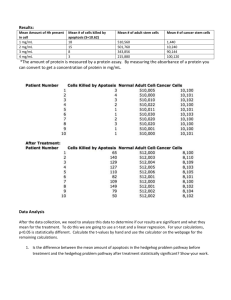
The Hedgehog-BMI1 Pathway The Hedgehog-BMI1 Pathway The Hedgehog (Hh) pathway regulates adult stem cell quiescence and self-renewal. Three Hh ligands have been identified in mammals, Sonic hedgehog (Shh), Desert hedgehog (Dhh), and Indian hedgehog (Ihh), of which Shh is the best studied. In the absence of ligand, the Hh receptor PTCH1 inhibits signaling through the catalytic inhibition of the transmembrane protein Smoothened (SMO). Ligand occupation of PTCH1 inactivates the receptor and allows activation of SMO that, in turn, results in the induction of Gli transcription factors; Gli1 and Gli2 are positive mediators of Hh signaling while Gli3 is a negative regulator.1,2 Many of the effects of Hh activation are facilitated by the induction of Bmi1, a polycomb gene that represses transcription through chromatin remodeling and down-regulates the expression of genes in the Ink-4A/ADP ribosylation factor (ARF) complex, such as p16 Ink4A and p19 ARF, that are negative regulators of the cell cycle and are involved in stem cell quiescence and differentiation.1,3 This enables stem cell proliferation and self-renewal via the Gli1- and Gli2-induced expression of the growth promoting genes cyclin D1, Myc, and Snail as well as upregulation of the Hh pathway elements PTCH1, Gli1, and Gli2.2,4 References 1. Liu, S., et al., Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res., 66, 6063-6071 (2006). 2. Peacock, C.D., et al., Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc. Natl. Acad. Sci. USA, 104, 4048-4053 (2007). 3. Massard, C., et al., Tumor stem cell-targeted treatment: elimination or differentiation. Ann. Oncol., 17, 1620-1624 (2006). 4. Mimeault, M., and Batra, S.K., Interplay of distinct growth factors during epithelial-mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann. Oncol., 18, 1605-1619 (2007).