Unit 1 & 2 Test Review
advertisement

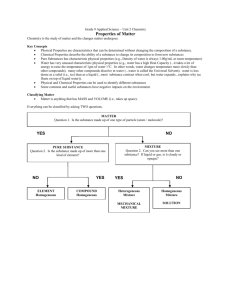
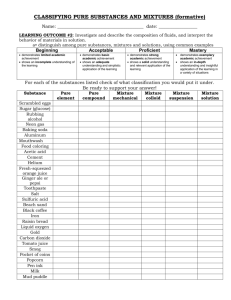
Unit 1 & 2 Test Review Rules of the game: Students will divide up into 4 groups. Each group will have one dry-erase board to record their answers. After the question slide is shown, the group will discuss the answer and one person will write on the dry-erase board. The first group to hold up the correct answer wins points for that question. READY???? Question 1 (10 points) True or False pH is considered to be a physical property of matter Answer: False; it is a chemical property because it is dependent upon the composition of the material Question 2 (15 points) A classroom lab experiment involved combining 2 different unknown substances. After combining, the students noticed that solid, white crystals formed in the bottom of the flask. What kind of change did the substances undergo? Answer: Chemical change; the white crystals were the result of the two substances chemically reacting Question 3 (20 points) Water was poured from a 500 mL beaker into three separate, smaller beakers. The first beaker had 20 mL, the second had 100 mL, and the third had 200 mL. Which beaker’s water had the highest density? Answer: the density is the same in all; density is an intensive property Question 4 (10 points) This safety symbol means what? Answer: biohazard Question 5 (15 points) Which state of matter is the most easily compressed? Answer: gas; its molecules are much further apart due to the high energy levels Question 6 (20 points) Definite shape and definite volume describe which state of matter? Answer: solid Question 7 (10 points) When ice melts, the density will decrease. Why? Answer: as the ice melts into water, the volume increases, but the mass stays the same (mass/volume) Question 8 (15 points) What piece of lab equipment is best for measuring mass? Answer: balance Question 9 (10 points) What is the biggest difference between a mixture and a pure substance? Answer: a mixture can be broken down (separated) into its components; a substance cannot Question 10 (20 points) Is an element considered to be a mixture or a pure substance? Answer: pure substance Question 11 (20 points) Is a compound considered to be a mixture or a pure substance? Answer: mixture Example: NaCl is sodium chloride; it can be separated chemically into Na (sodium) and Cl (chlorine) Question 12 (10 points) A change in state of matter (example: ice melting) is what type of change? Answer: physical Question 13 (20 points) A mixture that is uniform throughout with the same composition is called what? Answer: homogeneous Question 14 (20 points) Is water an element, mixture, or compound? Answer: compound Let’s look at water (H20) substances that cannot be “broken down” any further because it consists of water molecules and salt molecules. Question 15 (15 points) True or False Chocolate milk is an example of a heterogeneous mixture. Answer: false; it is homogeneous Question 16 (20 points) What is the density of an object with a mass of 5.09 g and a volume of 15 mL? Answer: mass/volume = 0.34 g/mL Question 17 (10 points) How do you dispose of chemical waste in the laboratory? A. pour it down the sink B. pour it back in the original container C. put it in a biohazard bag D. Follow the instructions given on the chemical’s MSDS Question 18 (15 points) Jim was working alone in the laboratory and dropped a beaker containing acid on the benchtop and it broke. What did Jim do incorrectly and what should he do now? The acid spill should be handled according to the MSDS (usually needs to be neutralized by a base). The glass should be carefully picked up and placed in a container set up specifically for broken glass; it should not be placed in the trash can. Question 19 (10 points) Bleaching hair would be what type of change? Answer: chemical