Introduction to Spectroscopy
advertisement
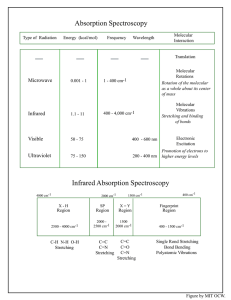
Introduction to Spectroscopy Illustrations from R. T. Morrison and R. N. Boyd, Organic Chemistry NMR Chemical Shift • Chemical Shift ~ measure of local electron density • = ( - ref )/ref * 106 ppm Peak Integrals • 3.8 : 2.9 : 8.8 = 1.31 : 1 : 3.03 = 3.9 : 3 : 9 • If formula is known C11H16 : • 16 H/15.5 units = 1.03 H per unit Correlation Charts 13C Spin-Spin Splitting • Multiplicity : singlet, doublet, triplet, quartet, pentet • Determined by number of near neighbors • N-Equivalent neighbors: multiplicity = N + 1 • • • • Branched Chains have Characteristic Patterns Quartet-triplet = ethyl Septet-doublet = gem-dimethyl (geminal – attached to same atom) Inequivalent Neighbors • N-Neighbors: multiplicity = 2N Spin Systems • Mutually coupled (or isolated equivalent spins) • Two spin systems Unknown • Double bonds and rings: CcHhOoNn • Dbr = (2c-h+n+2)/2 (2*8-10+2)/2 = 4 Effect of Field Strength • • • • Chemical Shift the same in ppm Multiplets have same spacing in Hz 60 MHz: 10 ppm = 600 Hz 1 Hz = 0.0166 ppm 500 MHz: 10 ppm = 5000 Hz 1 Hz = 0.002 ppm 500 MHz • Multiplets simplified (more first order) – Better definition in aromatic region • More sensitive (less sample or less time) Infrared Spectroscopy %T • Molecular Vibrations • Characteristic of functional groups • Chain Branching Aldehydes and Ketones • • • • • Aldehyde RCHO 1725 cm-1 Aromatic ArCHO 1700 cm-1 Conjugated -C=C-CHO 1685 cm-1 | Ketone R2CO 1710 cm-1 Aromatic ArCOR 1690 cm-1 -C=C-C=O 1675 cm-1 | | Cyclobutanones 1780 cm-1 Cyclopentanones 1740 cm-1 Alcohols Carboxylic Acids • • Aliphatic R-COOH 1700-1725 cm-1 Conjugated -C=C-COOH 1680-1700 cm-1 Aromatic Ar-COOH 1680-1700 cm-1 Carboxylic Acid Derivatives • Esters R-COOR C=O 1714-1740 cm-1 • two bands C-O-R 1050-1300 cm-1 Ethers Phenols • -CH2-CH2-CH2-CH2- rock 720 cm -1








![Synthesis and spectroscopic studies of some new oxazepine derivatives throughout [2+5]](http://s2.studylib.net/store/data/012450165_1-df99ca83df1aeeca39bbc9c3344ba468-300x300.png)