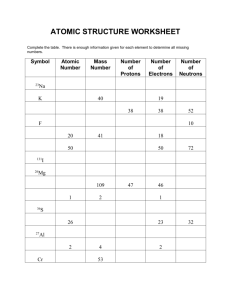
Atomic Mass Explained: Weighted Averages & Isotopes
advertisement
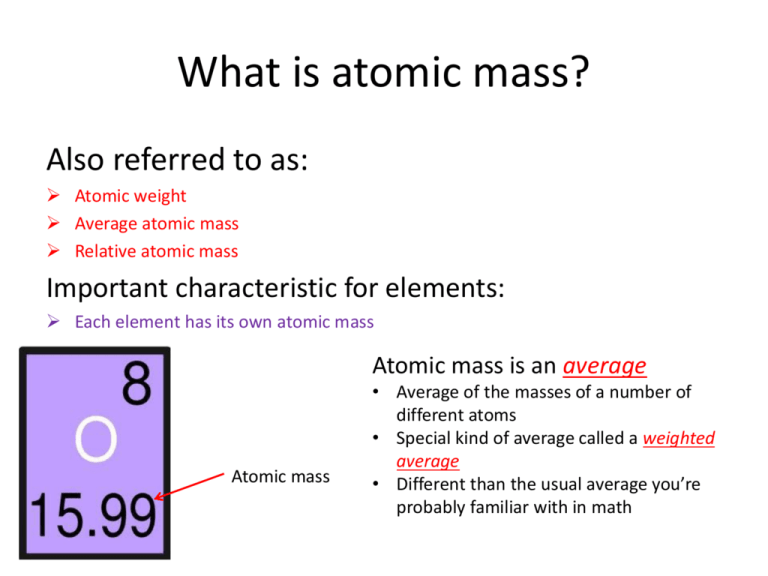
What is atomic mass? Also referred to as: Atomic weight Average atomic mass Relative atomic mass Important characteristic for elements: Each element has its own atomic mass Atomic mass is an average Atomic mass • Average of the masses of a number of different atoms • Special kind of average called a weighted average • Different than the usual average you’re probably familiar with in math Understanding Weighted Averages Even though these are different models and have different features, they are both lemonas due to their distinct lemon-like shape Using this analogy, the models of the lemona are similar to the isotopes of an element • 29 protons makes both atoms copper—even though they differ in their numbers of neutrons • Just like the “lemon-like” shape of a car makes it a lemona— even though they differ in their features Average vs. Weighted Average What is the average weight of the 2 cars? 4,000+5,000 • = 4,500 2 • Regular Average What would happen if we added in extra information? Weighted Average What is the average weight of lemonas, taking into account the amount of each model? 4,000 𝑥 .95 + (5,000 𝑥 .05) = 4,050 𝑙𝑏𝑠 Because there are so many more GXs than GXLs, the weighted average is much closer to the actual weight of a lemona GX Using Weighted Average with Different Atoms • Atomic mass: a weighted average of the masses for all the isotopes of a certain element Mass = 63 amu Mass = 65 amu If we pulled out a random sample of 100 copper atoms, we would find that 69% of them would be Cu-63 and 31% of them would be Cu-65 • 69% : Cu-63 • 31% : Cu-65 Atomic Mass of Copper 69% : Cu-63 31% : Cu-65 63 𝑥 .69 + 65 𝑥 .31 = 63.62 𝑎𝑚𝑢 Why are the 2 atomic masses different then? Difference between mass number and atomic mass Mass number: protons + neutrons 1 proton or 1 neutron = 1 amu • Therefore, if you have 6 protons and 6 neutrons, your atom is going to weigh 12 amu • If you have 6 protons and 7 neutrons, your atom is going to weight 13 amu Practice Gallium has 2 stable isotopes, and the masses of Gallium-69 (60.11% abundant) and Gallium-71 (39.89% abundant) are 68.926 amu and 70.925 amu, respectively. Calculate the average atomic mass of Gallium Practice Rubidium has 2 isotopes: Rubidium-85 (atomic mass of 84.911 amu) and Rubidium-87 (atomic mass of 86.909 amu). The atomic mass of Rubidium reported on the periodic table is 85.47 amu. Based on this information, which of the isotopes of Rubidium is more abundant? How do you know? • This is more of a thought problem. No real calculations are really necessary Practice Magnesium has 3 stable isotopes. Calculate its average atomic mass, using the information in the chart below. Isotope Mass Abundance Mg-24 23.985 amu 78.99 % Mg-25 24.986 amu 10.00 % Mg-26 25.983 amu 11.01 %