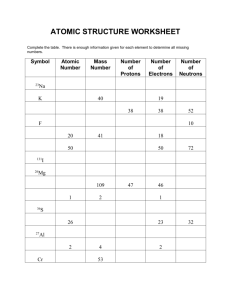
ISOTOPIC NOTATION isotopes are atoms with the same number of
advertisement

ISOTOPIC NOTATION isotopes are atoms with the same number of protons but different number of neutrons A Z X A = mass number (the total number of protons + neutrons) Z = atomic number (the total number of protons) X = element symbol READING ISOTOPIC NOTATION 46 21 Sc 46 = mass number (the total number of protons (21) + neutrons (25) 21 = atomic number (the total number of protons (21)) Sc = element symbol In a neutral atom, the number of electrons PRACTICE PROBLEMS 15N 7 8 7 # protons = ____ # neutrons= ____ #electrons = ___ 35P 15 # p = ____ 20 # n= ____ 15 #e- = ___ 33 # n= ____ 27 #e- = ___ 62Cu2+ 29 # p = ____ 76Se3- # p = 34 ____ 42 # n= ____ 37 #e- = ___ Writing ISOTOPIC NOTATION 1. Write the symbol for the atom with an atomic number of 21 and a mass number of 48. 48 Sc 2. Give the complete chemical notation for the nuclide with 23 protons, 26 neutrons and 20 electrons. 49V3+ 3. Write the isotopic notation for 110Pd a. Z = 46 A = 110 b. An atom containing 24 protons, 28 neutrons, and 21 electrons 52Cr3+ c. Titanium-50 50Ti PRACTICE PROBLEMS 1. Pt4+ 118 78 # n = _____ # p = _____ 196 196 mass number = ________ 195.1 amu atomic mass = ________ 74 #e- = _____ 78 atomic number = _______ platinum name of element = _______ 2. Indicate the appropriate atomic mass of an element with 30 protons, 30 neutrons, and 28 electrons. 65.39 amu Atomic Mass • The atomic mass of an element represents the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The atomic mass is calculated by adding the % of 1H mass found in nature to the % of 2H mass found in nature plus the % of 3H mass. • % 1H + % 2H + % 3H = average mass (atomic mass) • Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. Atomic Mass • Silver is found to have two stable isotopes, one has an atomic mass of 106.904 amu and the other weighs 108.905 amu. The first isotope represents 51.82 % of the mass of the element and the second represents 48.18 %. What is the atomic mass of the element silver? The equation to use is %X + % Y = average And remember to turn your percents into fractions before multiplying. (0.5182) 106.904 amu + (0.4818) 108.905 amu =? 55.398 amu + 52.470 amu =? 107.868 amu !! Now look at the periodic table to verify the answer. PRACTICE PROBLEMS # 8 1. A sample of neon contains three isotopes, neon-20 (with an isotopic mass of 19.9924 amu), neon-21 (20.9939 amu) and neon-22 (21.9914 amu). The natural abundances of these isotopes are 90.92%, 0.257 %, and 8.82 %. Calculate the atomic weight of neon. 20.17 amu 2. There are only two naturally occuring isotopes of copper, 63Cu and 65Cu. Copper has an atomic mass of 63.55 amu. What is the natural abundance of each 65Cu = 30% & 63Cu = 70% isotope? 3. There are only two naturally occuring isotopes of gallium, 69Ga and 71Ga. What is the natural abundance of each isotope? 69Ga = 60% and 71Ga = 40% GROUP STUDY PROBLEM #8 _______1. The element with atomic number 53 contains a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu 108 _______3. The number of neutrons in an atom of a) 47 b) 108 c) 155 47 Ag is d) 61 27 _______4. The number of electrons in an ion of a) 13 b) 10 c) 27 13 Al3+ is d) 14 _______5. What is the relative atomic mass of boron if two stable isotopes of boron have the following mass and abundance: 10.0129 amu (19.91%) & 11.0129 (80.09%) a) 10.81 amu b) 10.21 amu c) 10.62 amu d) 10.51 amu