Chapter 2 Notes
advertisement
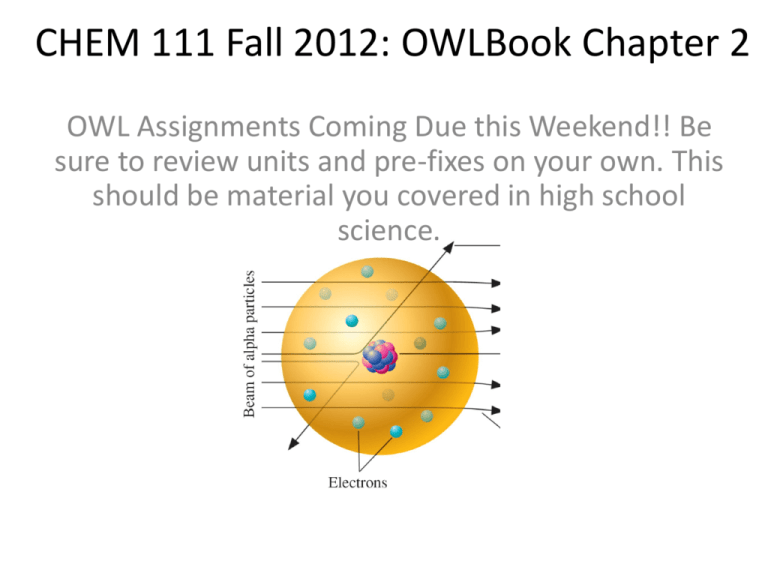
CHEM 111 Fall 2012: OWLBook Chapter 2 OWL Assignments Coming Due this Weekend!! Be sure to review units and pre-fixes on your own. This should be material you covered in high school science. Accuracy and Precision • Accuracy = how close you are to the known value • Precision = how reproducible (similar to each other) your results are X X X XX XX X X X X XX X X X Chapter 2: Elements and Compounds • Atomic Structure • Elements and Isotopes • The Periodic Table • Covalent Compounds – Naming • Ions and Ionic Compounds – Naming Atomic Structure • Protons: – Determine the identity of the element • Neutrons – With protons in the nucleus – Stabilize the nucleus (or make it unstable-radioactive) – Result in different isotopes • Electrons – Exist outside of the nucleus – Alter the overall charge on an atom • Adding or subtracting them makes ions Alpha Particle Scattering-helped determine the structure of the atom Subatomic Particles (for CHEM 111) Cathode Ray (electron beam) Tube Mass Spectrometer Atomic Symbols How many protons, neutrons, electrons are in: 65 30 Zn 76 33 ___(what is the element) Isotopes & Atomic Weight • Every form of a unique element is one of its isotopes – Some elements only have one isotope. • Remember-protons are what make an element • Isotopes are forms of an element with different masses • Adding or subtracting neutrons from the nucleus makes isotopes • Some isotopes are stable – Some are unstable • Radioactive Let’s look at some isotopes… Isotope Hydrogen Symbol 1 1 H Deuterium # of Neutrons ___________ Property/Use 0 “normal” hydrogen-stable isotope 1 Used to make “heavy water” 2 Radioactive. Used to make “glow in the dark” sights for firearms and other items (not cheap stuff) ___________ Tritium Fractional (isotopic) Abundance Atomic Weight What is the atomic weight of H ? Isotope Symbol 1 1 2 1 Hydrogen Deuterium Tritium 3 1 H H H # of Fractional Neutrons (isotopic) Abundance 0 1 2 The Periodic Table Groups, Periods, Main Group (A), Lanthanide & Actinide Elements, Metals, Non-Metals, Metaloids (semi-metals), Alkali Metals, Alkaline Earth Metals, Transition Elements (B), Halogens, Noble (“inert”) gases. What is an allotrope? Covalent Compounds • Compounds are formed when two or more elements join in a defined ratio • Covalent compounds consist of elements held together by covalent bonds – Covalent bonds are bonds where electrons are shared between atoms – The atoms don’t have a positive of negative charge on them • Some are named systematically, some have long used “historical” names. Empirical and Molecular Formula • Empirical formula has the ratio of atoms correct, but not necessarily the absolute number of each • HO • CF2 • Molecular formula has the absolute number of each atom in the molecule • H2O2 (hydrogen peroxide) • C2F4 (Teflon monomer) Types of Molecular Models Butanol • C4H10O1 • Wedge and Dash – Formula • Structural Formula • Ball and Stick Naming Covalent Compounds • Binary, non-metal compounds – Name first element (use prefix if needed) – Name second element, using a prefix if needed • Some have historical names that are more commonly used • H2O • CO • CO2 • UF6 • SiO2 • PF5 Naming Inorganic Acids (see trends?) (think about other names you may know for these) Ions and Ionic Compounds • Atoms normally have zero net charge – # of electrons = # protons • Ions have gained or lost electrons – Ions can form from any isotope – Ions have nothing to do with the nucleus, only the electrons. Some Monoatomic Ions What do the numbers mean? What is a cation? What is an anion? What trends or patterns do we see? Are these all the monoatomic ions there are? Polyatomic Ions (covalently bonded atoms that carry a charge amongst the whole group) Excuse me, Sir, but how many of these things are we going to have to know? My brain’s turning to Jell-O here! Formulas and Naming Ionic Compounds • Ions can have charge, but ionic compounds should have a net neutral charge • One can have Empirical and Molecular Formulas for ionic compounds • Instead of pre-fixes, IUPAC (International Union of Pure and Applied Chemistry) conventions are used. • Naming……. • The name of the cation (+) comes first. – If it has more than one possible charge, that number comes next • The name of the anion comes second. • CaF2 • LiF • Fe2O3 • Formulas – Remember, the charges MUST balance. • Use subscripts to make the charge balance. • Calcium Chloride • Zinc Sulfate • Calcium Nitrate • Magnesium Phosphate





