CH11_LG_U2_KEY - BC Learning Network
advertisement
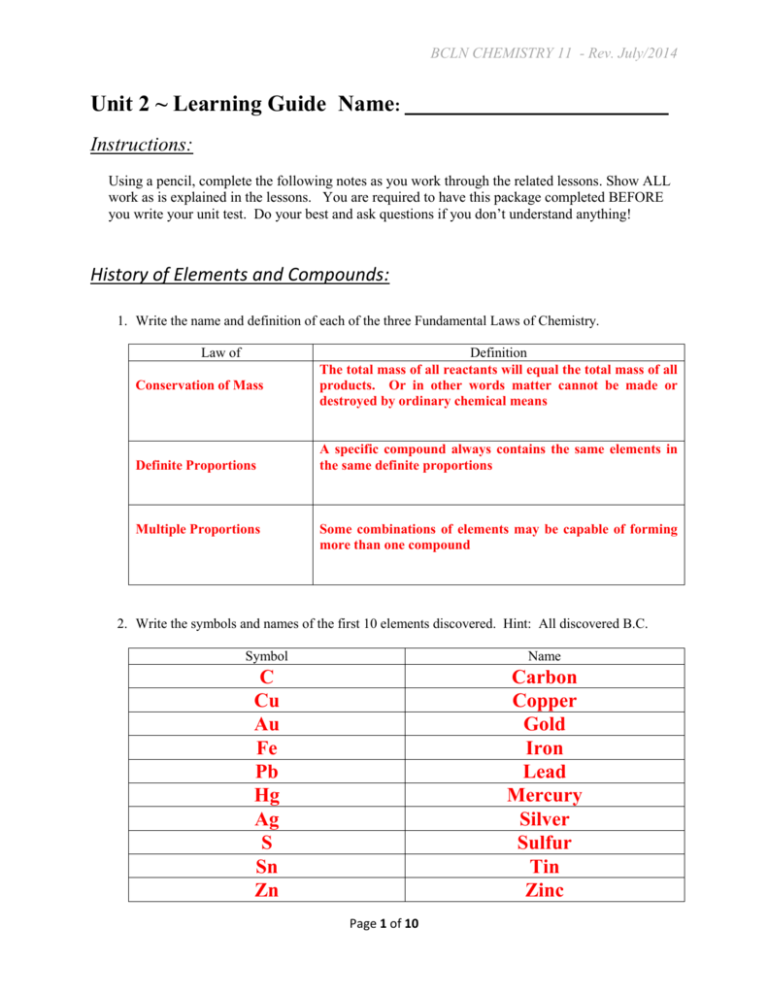
BCLN CHEMISTRY 11 - Rev. July/2014 Unit 2 ~ Learning Guide Name: ______________________________ Instructions: Using a pencil, complete the following notes as you work through the related lessons. Show ALL work as is explained in the lessons. You are required to have this package completed BEFORE you write your unit test. Do your best and ask questions if you don’t understand anything! History of Elements and Compounds: 1. Write the name and definition of each of the three Fundamental Laws of Chemistry. Law of Conservation of Mass Definite Proportions Multiple Proportions Definition The total mass of all reactants will equal the total mass of all products. Or in other words matter cannot be made or destroyed by ordinary chemical means A specific compound always contains the same elements in the same definite proportions Some combinations of elements may be capable of forming more than one compound 2. Write the symbols and names of the first 10 elements discovered. Hint: All discovered B.C. Symbol Name C Cu Au Fe Pb Hg Ag S Sn Zn Carbon Copper Gold Iron Lead Mercury Silver Sulfur Tin Zinc Page 1 of 10 BCLN CHEMISTRY 11 - Rev. July/2014 The Periodic Table: 1. Which scientist created the first periodic table and in what year did he do so? Dmitri Mendeleev in 1869 2. Why did his original periodic table have blanks in it? Some elements had not yet been discovered 3. What separates metals from non-metals on the periodic table? A "staircase" 4. Provide names for the following groups on the periodic table Group Name 1 Alkaline Metals 2 Alkaline Earth Metals 17 Halogens 18 Noble Gases 5. Which of these elements is more metallic, Titanium or Iron? Explain how you know this by discussing the position of the elements on the periodic table. Titanium because it is further to the left on the periodic table 6. Which of these elements, Fluorine or Carbon has a higher electronegativity? Explain how you know this by discussing the position of the elements on the periodic table. Fluorine because it is further to the right on the periodic table Page 2 of 10 BCLN CHEMISTRY 11 - Rev. July/2014 Periodic Table Groups 13-16: 1. What two well known materials are allotropes of carbon? Diamond and Graphite 2. Which element makes up 78% of the air we breathe? Nitrogen 3. Which element has an allotrope made from 4 atoms? Phosphorus 4. Which element has an allotrope made from 8 atoms? Sulfur Atoms, Molecules and Ions Classification: Atoms are made of 3 fundamental subatomic particles: protons, neutrons and electrons. The number of protons in a given atom is known as the atomic number and this number can be found on the periodic table. The number of protons and neutrons together is known as the atomic mass. The atomic mass on the periodic table is an average mass of different isotopes and should NOT be used for the following question. The number of electrons in an atom is equal to the number of protons. The charge of an atom is always zero. The charge of an ion is given by # protons - # electrons The full symbol for any given atom will tell you the atomic mass on the top and the atomic number on the bottom. Page 3 of 10 BCLN CHEMISTRY 11 - Rev. July/2014 1. Using the information on the previous page and your periodic table, fill in the following chart including BOTH symbol numbers. The first one is done for you. Symbol Atomic Number Atomic Mass p+ no e- charge H 1 2 1 1 2 -1 88 231 88 143 88 0 84 212 84 128 84 0 2 1 a) 231 b) 212 c) 35 17 Cl 17 35 17 18 17 0 d) 73 35 Br 35 73 35 38 35 0 e) 85 40 Zr 40 85 40 45 40 0 f) 107 47 Ag 47 107 47 60 47 0 g) 66 29 Cu 2 29 66 29 37 27 +2 F 9 18 9 9 10 -1 Ra Po h) 18 9 i) 127 53 I 53 127 53 74 54 -1 j) 36 17 Cl 17 36 17 19 18 -1 k) 27 3 13 Al 13 27 13 14 10 +3 2. List the diatomic elements & how can you plan to remember them. H2, O2, F2, Br2, I2, N2, Cl2 and the very rare At2 . One way is to think of a mysterious character called “HOFBrINCl” (Hoffbrinckel). Or make up a sentence as a mnemonic, e.g. Harry Often Needs Halogens. Page 4 of 10 BCLN CHEMISTRY 11 - Rev. July/2014 3. What are the elements that can be multiatomic? Show them in this form. P4, S8 and C (very long chains) Isotopes: 1. Put the following words in the correct blanks in the statement below : Mass protons Number neutrons Two isotopes of an element will have the same number of ____________protons________ but different numbers of _______neutrons_________. This means they will have the same Atomic _____Number______ but a different Atomic _____Mass_______. 2. a) Calculate the atomic mass of unknown element X given the precise atomic masses and the percent abundance of each isotope of the element X below X-13 12.98504 AMU 78.70% X-15 14.98584 AMU 10.13% X-16 15.98259 AMU 11.17% Atomic Mass = (12.98504 × 0.7870) + (14.98584 × 0.1013) + (15.98259 × 0.1117) Atomic Mass = 13.52 AMU b) Calculate the approximate atomic mass of potassium given this set of data: K - 39 92.36% K - 40 1.01% K - 41 6.73% Atomic Mass = (39 × 0.9236) + (40 × 0.0101) + (41 × 0.0673) Atomic Mass = 39.2 AMU Page 5 of 10 BCLN CHEMISTRY 11 - Rev. July/2014 3. For the following questions determine the percent abundance of each isotope. The periodic table is NOT needed for these questions and may even confuse you. Ideally you would use the algebra method shown in the course but if you find that confusing you can memorize the following formula instead. % 𝐨𝐨 𝐨𝐨𝐨𝐨𝐨𝐨𝐨 𝐨𝐨𝐨𝐨𝐨𝐨𝐨 = mass larger isotope − average mass x100% mass larger isotope−mass smaller isotope % of larger isotope = 100% - % of smaller isotope a) Oxygen - 16 and Oxygen - 18 with an average atomic mass of 16.2 Equation Method: % 16𝐨 = 𝐨𝐨 −𝐨𝐨.𝐨 𝐨𝐨𝐨𝐨% 𝐨𝐨−𝐨𝐨 = 𝐨𝐨% % 18𝐨 = 100% - 90% = 10% Algebra Method: Let x = decimal equivalent of abundance of 16O So 1-x = decimal equivalent of abundance of 18O Average atomic mass : 16 x 18 1 x 16.2 % 16O 100% 0.90 90% % 18O 100% 1.00 0.90 16 x 18 18 x 16.2 2 x 1.8 100% 0.10 2 x 1.8 x 0.90 % O 10% 18 b) An element has two isotopes, 52X and 54X, with mass numbers of 52.0 and 54.0 respectively. The relative atomic mass is 53.5. Equation Method: 54 53.5 0.50 100% 100% 0.25 100% 25% 54 52 2 % 54X 100% 25% 75% % 52X Algebra Method: Let x = decimal equivalent of abundance of 52X So 1-x = decimal equivalent of abundance of 54X Average atomic mass : 52.0 x 54.0 1 x 53.5 52.0 x 54.0 54.0 x 53.5 2 x 0.5 0.5 x 0.25 2 Page 6 of 10 % 52 X 100% 0.25 25% % 54 X 100% 1.00 0.25 % 54 100% 0.75 X 75% BCLN CHEMISTRY 11 - Rev. July/2014 Bohr and Quantum Theory: 1. State the FULL electron configuration of each atom. Be careful with Copper... Element Electron Configuration H 1s1 N 1s22s22p3 P 1s22s22p63s23p3 V 1s22s22p63s23p64s23d3 Cu 1s22s22p63s23p64s13d10 Br 1s22s22p63s23p64s23d104p5 2. State the electron configuration of each atom using core notation. Element Electron Configuration B [He] 2s22p1 S [Ne] 3s22p4 Co [Ar]4s23d7 I [Kr]5s24d105p5 Bi [Xe]6s24f145d106p3 Fr [Rn]7s1 Page 7 of 10 BCLN CHEMISTRY 11 - Rev. July/2014 3. Draw Bohr diagrams for the following chemical species. a) carbon atom b) neon atom --- 4 e- --- --- 8 e- --- --- 2 e- --- --- 2 e- --- 6 p+ 10 p+ c) aluminum atom d) calcium ion (Ca2+) --- 3 e- --- --- 8 e- --- --- 8 e- --- --- 8 e- --- --- 2 e- --- --- 2 e- --- 13 p+ 20 p+ Note that students may also draw diagrams with circles. Lewis Diagrams: 1. Define valence electrons? Those electrons in the outermost unfilled s and p orbitals of an atom 2. Explain how you can determine the number of valence electrons an element has? Count boxes from the edge of the periodic table until you reach the element. Do NOT count d orbital electrons! Or : For Groups 1-2 and 13-18, the number of valance electrons is the last digit of the group number, (except for He, which has 2 valance electrons rather than 8) Assume elements in Groups 3-12 have 2 valence electrons. Page 8 of 10 BCLN CHEMISTRY 11 - Rev. July/2014 3. Give the name and number of valence electrons for each of the following elements Element Symbol Element Name Number of valence electrons Be Beryllium 2 Ar Argon 8 Mn Manganese 2 Na Sodium 1 Br Bromine 7 Sb Antimony 5 4. Draw Lewis Diagrams for the following atoms and indicate the number of bonding electrons, number of lone pairs, expected number of covalent bonds each would make and the combining capacity. Bromine Hydrogen Nitrogen H Lewis Diagram # Bonding Electrons 1 1 3 # Lone Pairs 3 0 1 # Covalent Bonds 1 1 3 Combining Capacity -1 +1 or -1 -3 Page 9 of 10 BCLN CHEMISTRY 11 - Rev. July/2014 5. Draw Lewis diagrams & structural diagrams (no electrons) of the following molecules. For the last 3, the first element should be in the center with the other elements arranged around it. Molecule Br2 HCl CO2 NI3 N2 Lewis Diagram Structural Diagram Molecule Lewis Diagram Structural Diagram Answers: Isotopes: 2 a) 13.52 AMU 3 a) 16O = 90% b) 39.2 AMU 18 O = 10% b) 52X = 25% 54 X = 75% Page 10 of 10 CH2ClF




