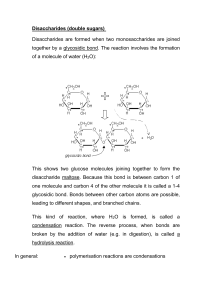
Carbohydrate Modeling Activity: Glucose & Maltose
advertisement

Name: ______________________________________ Carbohydrate modeling activity Purpose: To discover the elements that make up carbohydrates, one of the important biomolecules To demonstrate how monosaccharides can be combined through dehydration synthesis To demonstrate how monosaccharides can be created through hydrolysis PART A: Building a monosaccharide 1. Count your kit to make sure you have the correct number of the pieces necessary to create glucose a. ____ black pieces—represent carbon (can create 4 bonds) b. ____ blue pieces—represent oxygen (can create 2 bonds) c. ____ white pieces—represent hydrogen (can create one bond) d. ____ tubes—represent the bonds themselves 2. Set up the basic ring structure first. 3. Add all of the other elements to the ring to complete your molecule of glucose. 4. Once your molecule is completed, have your teacher check for accuracy. Then s/he will initial in the following space: ______________. PART B: Dehydration Synthesis ***For this part, you will be combining your glucose molecule with the glucose molecule of another nearby group*** 5. Lay the glucose molecules down so they have the same orientation (layout) as the picture in step 3 6. Remove the lone hydrogen from Carbon 1 on the left glucose molecule 7. Remove the bond and the OH group from Carbon 4 on the right glucose molecule 8. Reconnect the two glucose monomers 9. Make a water molecule out of the pieces that you removed 10. Your completed disaccharide, maltose, should look just like what you see below 11. Have your teacher check your maltose molecule for accuracy. S/he will then initial in the following space: ______________ PART C: Hydrolysis 12. Split the water molecule up into an –OH and H. Add these pieces back to the molecules from which you original took them. You will need to break up your disaccharide. Have your teacher check this last step for accuracy. S/he will initial in the following space: _______ Questions 1. How many of the follow elements are in the original molecule of glucose? a. Carbon _____ b. Hydrogen _____ c. Oxygen _____ 2. What would be the chemical formula for glucose (reminder: the formula for water is H2O). _________________________ 3. What is the simplified ratio of hydrogen to oxygen in a molecule of glucose? _______ 4. How does this ratio compare to the one you would find in water? _____________ 5. Why do we call glucose a “monosaccharide”? _____________________________________________ 6. Maltose was formed when we combined two monosaccharides together. What would be a good term to describe maltose? ____________ 7. Besides maltose, what was also produced when you joined the two monomers together? __________ 8. Why does the term “dehydration synthesis” make sense for what happened when you combined the two glucose molecules to make maltose? ____________________________________ ___________________________________________________________________________________________ 9. When you added the water back to the disaccharide, what happened to it? _______________ 10. In science, we use the terms anabolic to describe a process that builds something up and catabolic to describe a process that breaks something down. a. Is hydrolysis catabolic or anabolic? _____________________ b. Is dehydration synthesis catabolic or anabolic? _______________ 11. How many waters would I need to remove to join 5 monomers together? _________ 12. How many waters would I need to add to split a 7-unit polymer? __________