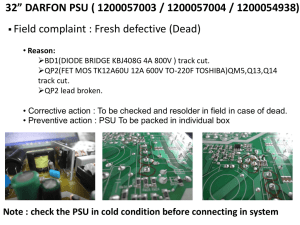
UnitsUncertaintyDimensionalAnalysis_Printable

Chem110 – Class Guide: Matter, Units, Uncertainty, and Dimensional Analysis (Chapter 1)
Chapter 1 Learning Goals:
Upon completion of Chapter 1, you should be able to:
Describe the relationships between matter and its microscopic component, including all of the general types of macroscopic and microscopic matter, and how they are related structurally.
Given the formula of a substance, identify it as a pure substance, a mixture, an element or a compound.
Identify the base units of the metric system and the prefixes commonly used. Be able to
convert from one common prefix to another.
Determine which numbers are significant and which are not in a given measurement. Apply the rules of significance to common calculations.
Apply the technique of dimensional analysis to various chemistry equations.
Chapter Reading Guide: Chapter 1 – INTRODUCTION: MATTER AND MEASUREMENT (You can skip the
‘Chemistry Put to Work’ sections throughout the text.)
Sections 2 & 3: CLASSIFICATIONS and PROPERTIES OF MATTER:
Know the states of matter and the differences between pure substances and mixtures
Identify pure substances, elements, mixtures (homogenous and heterogeneous), and compounds
View the Classification of Matter diagram
( http://www.bk.psu.edu/clt/chem110/matter.html
)
Section 4: UNITS OF MEASUREMENT:
Know the information in Table 1.4 for mass, length, time, temperature, and amount of substance
Memorize the following Prefixes in Table 1.5: Mega, kilo, centi, milli, micro, nano, pico
View the list of Metric System Prefixes
( http://www.bk.psu.edu/clt/chem110/metrix.html
)
TEMPERATURE:
Memorize how to convert from Celsius to Kelvin (Equation 1.1)
Section 5: UNCERTAINTY IN MEASUREMENT:
Understanding how many digits to include in a number is an important tool for chemists.
Try Sample Exercises 1.6, 1.7, and 1.8
Try this interactive exercise: Uncertainty in Measurement
( http://www.bk.psu.edu/clt/UncertaintyinMeasurement.swf
)
Listen to this podcast on significant figures: http://berks.psu.edu/chem/pod/SignificantFigures.mp3
Section 6: DIMENSIONAL ANALYSIS:
Read through Section 1.6 very, very carefully
Try all the Practice Exercises in the section. I know you can do most of these problems
without using dimensional analysis, but practice them using this technique!
TIP! As you work the problems, follow the book’s method exactly because the more familiar you get with dimensional analysis, the easier the course will be for you! Almost everything we do from here is dimensional analysis in one form or another, but we call it something different.
Learning Resources:
Chapter Learning Goals:
Interactive Exercises:
Uncertainty in Measurement ( http://www.bk.psu.edu/clt/UncertaintyinMeasurement.swf
)
Additional Chapter Resources:
Classification of Matter Diagram ( http://www.bk.psu.edu/clt/chem110/matter.html
)
Metric System Prefixes ( http://www.bk.psu.edu/clt/chem110/metrix.html
)
Podcast Significant Figures ( http://berks.psu.edu/chem/pod/SignificantFigures.mp3
)
End of Chapter Exercises: Practice with these problems if you are having difficulty with any of the concepts covered in this class guide AFTER we have met in class. If you cannot easily complete these problems, seek help from your instructor, your mentor, or the learning center.
Chapter 1: 11, 15, 25, 27, 35, 39, 45, 47, 49, 53, 65