Determining the Density of Unknown Liquids
advertisement

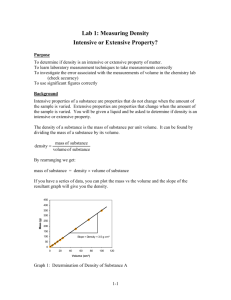
Determining the Density of Unknown Liquids Pre-Lab Questions: 1. What is an intensive property? List five intensive properties. 2. What is the density of water? What is the mass of 10 ml of water? 3. Using the concept of density, explain how metal boats are able to float on water. Background: One of the most important intensive properties of matter is the density. Intensive properties are those that do not depend on the amount of the substance present. These are especially important to help determine the identity of a substance. In other words, the density of a substance can be crucial in determining the identity of an unknown substance. Here is a table of some common substances and their densities. Density Table Substance air ice water sea water ethanol aluminum glycerol iron silver Isopropyl alcohol Density g/cm3 0.0013 0.917 1.00 1.03 0.790 2.70 1.261 7.86 10.50 0.786 Purpose: Determine the identity of three unknown liquids based on your calculations of density. Safety information: Whenever you are dealing with substances you cannot identify, you must exercise extreme caution. Wear your goggles at all times during this lab and wash your hands thoroughly and often. Materials 1 250 mL beaker 1 100 mL graduated cylinder Calculator Substances A, B, C Procedure: 1. 2. 3. 4. 5. 6. 7. Pour approximately 100 ml of substance A into a 250mL beaker. Use a graduated cylinder to measure 10 mL of Substance A. Measure the mass of the 10mL of Substance A. Determine the density of this amount of the substance. Repeat steps 2-4 using 20mL of Substance A. Repeat steps 2-4 using 30mL of Substance A. Return Substance A to the appropriate “Source beaker” and rinse your 250mL beaker thoroughly. 8. Pour approximately 100 mL of Substance B into your 250 mL beaker. 9. Repeat steps 2-7 using Substance B. 10. Again repeat steps 2-7 but now use Substance C. Questions to Consider (Conclusion) 1. Use the density table on this lab to identify your three substances. Verify your guesses with your instructor. 2. Determine the percentage error of your density calculations. The formula for percent error is as follows: % error = (|Your Result - Accepted Value| / Accepted Value) x 100. Please calculate this for each of your substances.