Specific Heat Capacity
advertisement

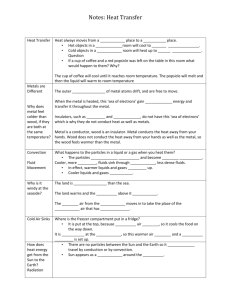
Name _________________________ Date __________ Partners ___________________________________________________ APHY101 – Lab – Heat Introduction Heat is the flow or movement of thermal energy from one point to another. In lab, we would like to measure this flow of thermal energy when two objects are brought together. However, since there is no practical way to measure this flow of thermal energy, we have to resort to an indirect method of doing so. This is accomplished by measuring the change in temperature of the objects and then determining the change in energy: Q = mcT Eq.1 In the above equation, Q is the thermal energy necessary to change the temperature T of an object of mass m which has a specific heat capacity of c. You may think temperature and heat mean the same thing, however this is not the case. Two objects may both experience a rise in temperature of 5C but the change in thermal energy for each object may be quite different. If two objects at different temperatures are brought together, thermal energy will be lost by the hotter object and gained by the cooler one. Now, if the two objects are mixed in an isolated container so that the effects of outside temperature can be ignored, the Law of Conservation of Energy can be applied. This law states that for an isolated system the total energy in the system remains constant. Energy may be transferred around in the system but is never created nor destroyed. We can use Equation 1 to determine one of the variables. m m c m Δ Tm = m w c w Δ T w Eq. 2 Looking more closely at the T terms, you will see that Tm = Tm - Tf Tw = Tf - Tw where Tf is the final or equilibrium temperature of the metal and water. If we know the specific heat capacity of the water, we can find the specific heat capacity of the metal by dividing Equation 2 by mm (Tm – Tf ): cm = m w c w (Tf - Tw ) m m (Tm - Tf ) Eq. 3 Equipment metal samples, hot plate, calorimeter, thermometers, beaker, string, stopwatch, PASCO light apparatus, ink, power supply, cardboard tube with lead pellets, CBL thermometer 1 Neil E. Miller 3/9/2016 Procedure Part A: Specific Heat Capacity All masses in kg and temperatures in °C 1. Begin heating some water in the beaker on the hot plate until the water boils. CAUTION!!! 2. Determine the mass mm of each metal given to your group and the mass of your cup mc. 3. Once the water is boiling, carefully place one of the metal samples into the beaker using a loop of string. Use a thermometer to measure the temperature of the water. The metal should remain in the water for 5 minutes. 4. While the metal is heated, fill the cup about 1/3 full with cold water from the water fountain. Weigh the cup and water and determine the mass of the water mw. Place the other thermometer in the water. When the metal is thoroughly heated, record the temperature of the metal Tm and the initial temperature of the water Tw. Now quickly but carefully add the hot metal to the cup. Replace the cup's lid on as soon as possible and then allow the mixture to reach a constant temperature. Record the final temperature of the mixture Tf. While waiting for the first metal to reach Tf, begin heating another metal. 5. Repeat for the other metal samples with fresh water in the cup and complete the data table. 6. Repeat for the metal sample with the largest percent error. mc = __________ kg Type of metal mm mw cw = 4186 J/kg °C Tm Tw Tf cc = 920 J/kg °C Experimental Accepted cm cm Aluminum 920 Brass 385 Iron 460 Lead 130 2 Neil E. Miller 3/9/2016 % error in cm 1. Identify several sources of experimental error and describe ways to decrease their effect. 2. If the metal is wet when added to the water in the cup, how would this affect the experimental value of the sample’s specific heat capacity? 3. Which metal should have the largest percent error? Why? Was this your metal? 3 Neil E. Miller 3/9/2016 Part C: Heating with a Light Bulb 1. Fill each cup to the line with water and place the same number of ink drops in each cup. Take the temperature of the water and then connect the light to the power supply. Set the voltage to 11 volts and run each light with and without the Styrofoam cup for 10 minutes. 2. Record the temperature change and add your data to the class graph. Number of drops = ______ Initial Temperature = ________ °C ΔT with cup = _________°C ΔT without cup = _________°C Explain the class results based on the number of ink drops used and the affect of the Styrofoam cup. Part D: Does GPE = Q? 1. Take the temperature of the lead with the CBL thermometer. Tip the tube over 100 times then quickly take the temperature again. Initial Temperature = _______ °C Final Temperature = _______ °C ΔT = _________ °C 2. Calculate the gravitational potential energy of the lead: GPE = mass of lead*acceleration of gravity*total distance the lead travels = __________ J 3. Calculate the thermal energy transferred to the lead: Q = mass of lead*specific heat of lead* ΔT = __________ J 4 Neil E. Miller 3/9/2016 4. Does GPE = Q? If not, which is larger and why? 5. Which method(s) of thermal energy transfer is most important during this experiment: conduction, convection or radiation? Explain. 6. Why did you use lead for this experiment instead of one of the other metals? 5 Neil E. Miller 3/9/2016