Investigating the Properties of Water
advertisement

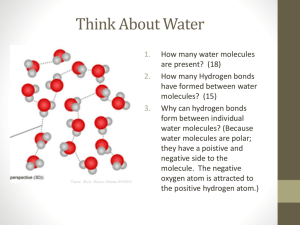
Name ____________________________________________________ Date ___________ Hour ___________ Water: Structure and Properties Activity 1: Gathering background information 1. Turn to page 156, and read about the importance of water. a) Describe several reasons why water is important. 2. Read “Water is Polar”. a) Define Polar Molecule (record the entire definition) - b) Define Hydrogen Bonding - c) What charge (positive or negative) does the hydrogen have? ____ Oxygen? _____ *Polarity and Hydrogen bonding give water some of its special properties, we are learning 3 of them. 3. Read the last paragraph under “Water is Polar” on page 157 and describe capillary action. Property 1: Capillary Action Property 2: Surface tension – property of a liquid that makes its surface behave like an elastic skin. Property 3: Cohesion – water sticks together in droplets (water is attracted to other water molecules) Activity 2: Investigating the Special Properties of Water Station A: Pennies 1. How many drops of water do you think will fit on the head of a penny? Make a hypothesis 2. Using a dropper slowly drop water onto a penny counting each drop. How many drops of water did fit on the head of a penny? ______ 3. Draw what the penny looks like, as viewed from the side, before it overflowed. 4. This activity illustrates 2 of the 3 properties of water that you need to know. List the two and describe how it shows them. 5. Clean the station and dry the penny!!! Station B: Wax Paper 1. Place several drops of water on a piece of wax paper. Describe what happens to the water droplets as you roll them around on the wax paper? 2. Which of the three properties of water does this best show? Why? 3. Clean the station and dry the wax paper Station C: Paper Clips 1. Fill a beaker or cup till it is just about to overflow. Add water until it rises over the edge of the beaker, but does NOT overflow. How is it that water can be higher than the lip of the beaker? Use one of the properties of water in your answer. 2. Balance a paper clip on the surface of water,.(hint: don’t let your fingers touch the water) a) Describe how the water looks around the edges of the paper clip. b) Use scientific reasoning to explain why it looks this way. 3. Empty the beaker and place paper clip back on tray. Station D: Structure of Water 1. Use the model kit to create the structure of water. Use figure 6.12 from the book on page 157 to help you to do this. Draw and color the model below. a) Label the Hydrogen’s and the Oxygen by writing the H (for hydrogen) and O (for oxygen) b) Write in the charges (+ = positive, and - = negative) of the hydrogen’s and the oxygen next to their letters in the drawing. c) Describe the shape of the water molecule d) Now draw in another water molecule (above) with all parts labeled like you did in parts A and B. Place it where you think it should go considering that “Opposites attract.” Station E: Hydrogen Bonding analogy 1) Using the two magnets. Describe what happens when the red half of one touches the black half of another. 2) Now, try and touch two ends of the same color. Describe what happens. 3) Using figure 6.12 on page 157 as a guide, how is this a good analogy for demonstrating how a water molecule behaves when it comes in contact with another water molecule? a) What is the special type of bond called between two water molecules? ________________ b) Why/how does this bond form? Activity 3: Analysis 1. Considering Stations D and E: How does the structure of water relate to how water molecules interact with each other? Use scientific reasoning in your answer. 2. What are the three special properties of water that you learned about? 3. How does polarity and hydrogen bonding lead to these special properties of water? 4. Classify the following as cohesion, surface tension, or capillary action. a) b) c) d) ______________ An insect walking on the surface of water. ______________ dew drops collecting on blades of grass. ______________ water going up a tree to reach the leaves at the top. ______________ rain drops 5. How do you think the human body uses capillary action in the body? Provide at least two examples where this would occur in the human body. 6. How do you think the human body uses adhesion? Give an example. 7. How do you think the human body uses cohesion? Give an example.