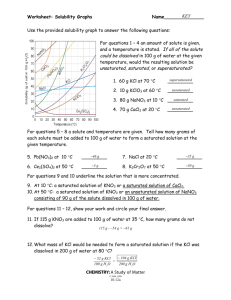
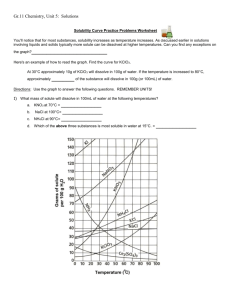
Solubility Curve Practice Problems Worksheet 1
advertisement

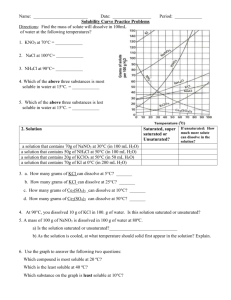
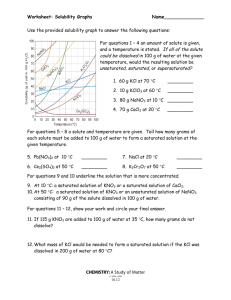
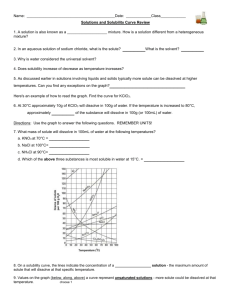
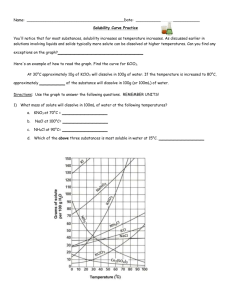
Name: __________________________________________ Solubility Curve Worksheet and Lab We are using the solubility graph (Table G) in this lab. Note the following: - Solubility (grams of solute in 100 mL of water) is on the left - Temperature is on the bottom - Note that solids always go up (more goes in as we add temperature) - Note that gases always go down (less goes in as we warm it up – like your soft drink) - You need to know 3 terms: Unsaturated – more can go in (below the line) Saturated – as much as it can hold (on the line) Supersaturated – more than it can hold (above the line) - If a question asks 200 g of water than you double the answer. If 50 than half it. - If you need to figure out how much more you can add or how much comes out, just subtract the difference. Here's an example of how to read the graph. Find the curve for KClO3. At 30°C approximately 10g of KClO3 will dissolve in 100g of water. If the temperature is increased to 80°C, approximately ______ of the substance will dissolve in 100g (or 100mL) of water. At 30 o C – 12 g at 80 o C – 44 g so answer: 32 g Note that KCLO3 is a solid because it goes up. Here’s an 2nd example of NH3. Is 20 g NH3 saturated, unsaturated, or supersaturated at 60 o C? Answer: Unsaturated – below the line. You would need to add 3 more grams to be saturated. Note: NH3 is a gas – the line goes down! Directions: Use the Table G to answer the following questions. REMEMBER UNITS! 1) What mass of solute will dissolve in 100mL of water at the following temperatures? a. b. c. ____________ NaCl at 100°C= ____________ NH4Cl at 90°C= ____________ KNO3 at 70°C = d. Which of the above three substances is most soluble in water at 15°C. = ____________ 2) Types of Solutions On a solubility curve, the lines indicate the concentration of a __________________ solution - the maximum amount of solute that will dissolve at that specific temperature. Values on the graph ____________ a curve represent unsaturated solutions - more solute could be dissolved at that temperature. Values on the graph ____________ ac curve represent saturated solutions which has more than it can hold. Label the following solutions as saturated or unsaturated. If unsaturated, write how much more solute can be dissolved in the solution. Solution Saturated or Unsaturated? If unsaturated: How much more solute can dissolve in the solution? a solution that contains 70g of NaNO3 at 30°C (in 100 mL H2O) a solution that contains 50g of NH4Cl at 50°C (in 100 mL H2O) a solution that contains 20g of KClO3 at 50°C (in 100 mL H2O) a solution that contains 70g of KI at 0°C (in 100 mL H2O) _______ What is the solubility of KCl at 25C? _______ What is the solubility of Ce2(SO4)3 at 10C? _______ What is the solubility of Ce2(SO4)3 at 50C? _______ 1. a. What is the solubility of KCl at 5C? b. c. d. 2. a. At 90C, you dissolved 10 g of KCl in 100. g of water. Is this solution saturated or unsaturated? b. How do you know? 3. A mass of 100 g of NaNO3 is dissolved in 100 g of water at 80ºC. a) Is the solution saturated or unsaturated?______________________________ b) As the solution is cooled, at what temperature should solid first appear in the solution? Explain. 4. Use the graph to answer the following two questions: ________ ________ Which compound is most soluble at 20 ºC? Which is the least soluble at 40 ºC? 5. Which substance on the graph is least soluble at 10C? __________ 6. A mass of 80 g of KNO3 is dissolved in 100 g of water at 50 ºC. The solution is heated to 70ºC. How many more grams of potassium nitrate must be added to make the solution saturated? Explain your reasoning 7. Use the graph to answer the following two questions: Which compound is most soluble at 20 o C? ______________________ Which compound is least soluble at 40 o C? __________________ 8. What are 2 gases on the graph? ___________________________________________ How do you know? 9. A mass of 80 g of KNO3 is dissolved in 100 g of water at 50 o C. The solution is heated to 70 o C. How many more grams of potassium nitrate must be added to make the solution saturated? Show your work. 10. How many grams of potassium nitrate must be added to 100 grams of water to make a saturated solution at 50 0 C? 11. How many grams of potassium chlorate must be added to 50 grams of water at 50 0 C to make a saturated solution? 12. A saturated solution of sodium nitrate in 200 grams of water is cooled from 60 How many grams of precipitate are formed? 0 C to 10 0 C.