Experiment #9: Boyle's Law
advertisement

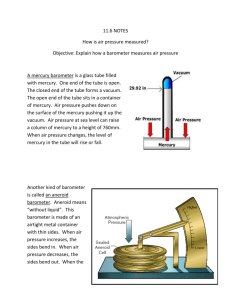
Experiment 9: Boyle’s Law Purpose (1) To verify Boyle’s law for an Ideal Gas; (2) To determine the atmospheric pressure. Apparatus A capillary tube with a pocket of air trapped by a column of mercury; a meter stick. Theoretical Summary: Boyle’s Law states that a fixed amount of gas, V, kept at constant temperature, T, changes its volume (if the pressure is changed) according to the equation: P · V = constant (1) In this experiment a capillary tube (Fig. 1) contains a pocket of gas of length l trapped by a column of mercury of length L. The length l changes depending upon the angle of the tube. The length of the mercury L, is approximately constant throughout the experiment. 43 Experiment 9 The pressure P inside the capillary tube must balance the atmospheric pressure Po plus the pressure caused by the weight of the mercury column. The pressure on the gas pocket due to the weight of the mercury column is the component of the weight which is pointing in the direction of the gas pocket. From trigonometry, the component pointing in this direction is the weight times cos θ. So the pressure from the mercury column is the density of mercury volume of the column cos θ = gL · cos θ. Then P = Po + gL · cos θ (2) where = density of mercury, and g = acceleration of gravity. If the cross-sectional area of the tube is A, then the volume of the gas pocket is V = A · l and then Boyle’s Law (Eq. 1) can be expressed as (Po + gL · cos θ) · Al = constant = C (3) Although we do not know some the constants Po, A, and C, in equation (3), there are only two variables (l and θ) which are of interest in this experiment. The goal is to verify this equation by constructing a convenient graph based on the measurements of l for a given θ. Instead of measuring the angle θ directly, we use two loaded strings as plumb lines (See Fig. 2). and calculate: cos = H / d = (d2 - S2) / d = 1 - (S/d) 2 44 (4) Experiment 9 Procedure: (a) Measure L and d and record. Prepare a table to record the values of S and l for various positions of the tube. (b) Start with the tube in vertical position, open end up. Record l and S on your data sheet. (c) Tilt the tube slightly so that S is equal to several cm and record l and S again. (d) Repeat (c) several times by tilting the tube more and more until it becomes horizontal ( = 90). You can locate the horizontal position exactly (by making S equal to d) and include this data in your table. (e) Continue tilting the tube (because of capillarity effects, the mercury will not spill out). Repeat (c) several times more, until the tube is now vertical ( = 180), but with the open end down. Lab Report Part I. Boyle’s Law (A) Show that, by introducing new variables: x = L · cos θ, and y = 1/l , Eq. (3) can be recast in the form of a straight line, namely: y = mx + b (5) Calculate m and b in terms of the other parameters. What are the units of m and b? (B) From your data, calculate the values of x and y for each position of the tube. Don’t forget to put the units in the table header. S l (S/d)2 1 - (S/d)2 cos θ = 1 - (S/d)2 Important: Use the appropriate sign for cos θ, meaning: cos θ > 0 cos θ < 0 for for 0 < θ < 90, and 90 < θ < 180. 45 x = L· cos θ y =1/l Experiment 9 (C) Plot y vs. x on a sheet of graph paper, utilizing the entire sheet. (See Fig. 3.) (D) State clearly whether or not your plot verifies Boyle’s Law, and why. In case it does not, state clearly what went wrong. Part II. Measuring Po. (E) According to your work under (A), the ratio b/m should be equal to Po/g. From your graph in Fig. 3, measure the slope m and the y-intercept b and calculate Po. The density of mercury is 13.5 g/cm3. (F) Compare with the accepted value of Po (express your results in the same units) and comment on the discrepancy. (Po is approximately 1.01 106 g/cm-s2) Question: If θ > 90°, is P larger or smaller than Po? Explain why. 46