tests%20for%20FLAVONOIDS
advertisement

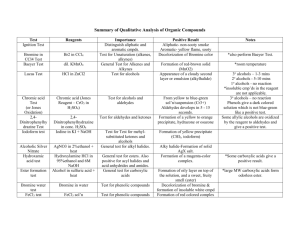
CHAPTER III GENERAL TESTS First Test for Flavonoids 1. A small amount of the collected filtrate or corn extract was put in a test tube 2. 5 gtts. of 5% NaOH was added to the extract followed by addition of 2mL 10% HCl. 3. A yellow solution that turns colorless upon addition of HCl indicates the presence of flavonoids. The flavonoids being tested in the corn extract contain polyphenolic molecules which are weakly acidic in nature. In the first general test for flavonoids, the extract was first treated with a small amount of a weak concentration of NaOH (5%). The addition of 5% NaOH to the extract didn’t give very evident reactions because at first, it only gives a colorless solution which contains sodium phenoxide. Also, only 5 gtts of a weak concentration of NaOH was added. (G.A. Ayoola, September 2008) NaOH is completely ionic, containing sodium cations and hydroxide anions. It is a sufficiently strong base which deprotonates phenol entirely. The purpose of 5% NaOH in this test was to deprotonate the polyphenolic molecules cointained in flavonoids. NaOH turns phenols into phenoxides which are much more soluble in water than phenols. The alkaline condition may have been necessary to disrupt bonding between the flavonoids and other components of the brain. (G.A. Ayoola, September 2008) Flavonoids give the yellow coloration to the extract. The objective of this experiment was really to decolorize the yellow color of the corn extract containing flavonoids. This was done through the addition of HCl to the mixture of the corn extract and 5% NaOH. Only a minimal amount of NaOH was put in the extract to avoid the tendency of HCl to neutralize with the mixture. The addition of sufficient amounts of hydrochloric acid prevents the development of colored complexes of many phenols and all enols. This is the reason why upon addition of HCl to the mixture, the solution slowly decolorizes. (G.A. Ayoola, September 2008) The experimental result of the first general test for flavonoids was consistent to its theoretical positive result which is the decolorization of the extract. This indicates the presence of polyphenolic flavonoids in the corn extract. (G.A. Ayoola, September 2008) Third Test for Flavonoids 1. A portion of the extract was heated with 10 ml of ethyl acetate over a steam bath for 3 minutes. 2. The mixture was filtered and 4 ml of the filtrate was shaken with 1 mL of dilute ammonia solution. 3. A yellow coloration indicates the presence of flavonoids. This general test for flavonoids involves the addition of ethyl acetate to a portion of the corn extract. The mixture was subjected to heat for 3 minutes in a water bath and then it was filtered. The corn extract is suspected to contain polyphenolic flavonoids. The ethyl acetate which is an ester reacted with these flavonoids by decolorizing it upon subjection to heat. With the addition of dilute ammonia solution, an amine, to the phenyl ester, the yellow coloration of the extract was recovered indicating the presence of flavonoids. (G.A. Ayoola, September 2008) Fourth Test for Flavonoids 1. 4mL of aqueous NaOH was added to 2ml of each of ethanol extract. 2. Formation of a yellow precipitate indicates the presence of flavonoids in the extract. Flavonoids are soluble in NaOH solution since the former contains weakly acidic polyphenols and the latter is a strong alkaline medium. When phenol is reacted with aqueous NaOH, it gives a colorless solution which contains sodium phenoxide. The hydroxyl part of phenol loses H+ forming phenoxide ion and H2O. Though the negative charge of phenoxide ion is now delocalized around the ring, electrons are drawn towards the oxygen because of its electronegativity. (T.I. Olabiyi, 2008) Since aqueous solution of sodium hydroxide is strongly alkaline due to its complete dissociation into Na+ and OH-, sodium cation is attracted by the oxygen of phenoxide ion. That’s why at first, formation of colorless globule-like sodium phenoxide in the sample is seen. But after some time, yellow precipitate accumulated. This is due to the fact that phenol is fairly insoluble in H2O. While some phenols solubilized with NaOH, some also reacted with the H2O produced by the first reaction which is characterized by the late formation of yellow precipitate. (T.I. Olabiyi, 2008) SPECIFIC TESTS Chromic Acid Test or Jone’s Test 1. Dissolve 1 gtt. of the extract or a small amount of the solid sample in 1 mL of acetone in a small vial. 2. Add 2 gtts. of 10% aqueous K2Cr2O7 solution and 5 gtts 6M H2SO4 . 3. The appearance of the green Cr3+ ion or a blue-green solution indicates oxidation of the compound or the presence of primary or secondary alcohols in the sample. Chromic acid is capable of oxidizing many kinds of organic compounds. In organic chemistry, dilute solutions of hexavalent chromium can be used to oxidize primary or secondary alcohols to corresponding aldehydes and ketones while tertiary alcohols are unaffected. Oxidation is signaled by a color change from orange to a bluegreen coloration. The principle involved in the Jone’s test is oxidation. Oxidation is a qualitative analytical test for the presence of primary or secondary alcohols in a sample. Oxidation is readily detected by the appearance of the green Cr3+ ion or a blue-green solution. Primary and secondary alcohols are rapidly oxidized by chromium trioxide in acidic, aqueous acetone, whereas tertiary alcohols are stable to oxidation. Chromic acid in aqueous H2SO4 and acetone is known as the Jone’s reagent which oxidizes primary and secondary alcohols to carboxylic acids and ketones respectively, while rarely affecting unsaturated bonds. Primary and secondary alcohols and aldehydes react with chromic acid or Jone’s reagent and give a blue-green precipitate in this test. Aldehydes are oxidized to carboxylic acids, secondary alcohols are oxidized to ketones and primary alcohols are oxidized first to aldehydes and then to carboxylic acids. Ketones and tertiary alcohols are unreactive under the test conditions. The polyphenolic flavonoids in the extract contain secondary alcohols in its chemical structure which caused the rapid oxidation of the compound by chromic acid and the formation of a blue-green solution. The experimental result of Jone’s test to the corn extract is consistent with the theoretical positive result which proves that the prepared corn extract contains oxidizable secondary alcohols. Lucas’ Test Lucas’ Reagent: Dissolve 16 grams of anhydrous zinc chloride in 10 mL of conc. HCl with cooling. 1. Add about 50 mg or 2-3 gtts of the extract to 1 mL of the reagent in a small vial. Cap the vial and shake vigorously for a few seconds. 2. Allow to stand at room temperature. Turbidity indicates the presence of alcohol compounds in the sample. Tertiary alcohols react immediately with Lucas’ reagent to produce turbidity while secondary alcohols do so in five minutes. Primary alcohols do not react appreciably with Lucas reagent at room temperature. Coordination of the zinc chloride with the hydroxyl results in the formation of a sufficiently good leaving group. The carbon-oxygen cleavage can occur when reasonably stable carbocation is produced. Alkyl Chloride (insoluble-cloudiness) Lucas’ test for alcohol is a test to differentiate primary, secondary and tertiary alcohols. It is based on the difference in reactivity of the three classes of alcohols with hydrogen halides. The principle behind this is the SN1 or substitution nucleophilic by first order reaction which caused the turbidity (halide compounds insoluble in water.) The experimental result is the formation of a turbid solution in a few minutes which is consistent with the theoretical result of Lucas’ test for secondary alcohols. This indicates the presence of secondary alcohols in the corn extract. Bromine Test The bromine test is a qualitative test for the presence of unsaturated C–C bonds and phenols. 1. The sample is treated with a small amount of elemental bromine — either as an aqueous solution, or as a solution in dichloromethane or carbon tetrachloride. 2. A positive test for the presence of unsaturation and/or phenol is indicated by the disappearance of the deep brown coloration of bromine, which happens because the bromine has been consumed by reaction with the unknown sample. 3. The formation of a white precipitate indicates the formation of a brominated phenol. The decolorization of bromine water is often used as a test for the presence f a carbon-carbon double bond. The addition of bromine to cyclohexene gives a racemic trans product. Since the product’s color differs from the original deep brown solution, the bromine is rapidly decolorized when added to an alkene. When bromine water is decolorized, there is a presence of unsaturated fatty acid. Alkenes and alkynes will readily add bromine across the multiple bonds unless there are electron withdrawing groups on the multiple bonds. One observes the rapid disappearance of the red-brown bromine color. Aromatic compounds can react with bromine more slowly to give bromine substitution and the formation of HBr, which can sometimes be observed by placing a piece of wet litmus paper over the mouth of the test tube. The principle behind this reaction is the addition of a halogen into the double bond. These results to an alkene with a halogen attached. The experimental result is the same with the theoretical result where there is the formation of white precipitate due to the discharging of the bromine color without the evolution of the hydrogen bromide gas. Baeyer’s Test The Baeyer’s test for unsaturation is for determining the presence of carbon-carbon double bonded compounds, called alkenes or carbon-carbon triple bonded compounds, called alkyne bond. 1. Place 5 gtts. of sample in a dry test tube. 2. Add 2 gtts. of 2% KMnO4 solution. Shake the test tube vigorously and observe the rate and extent by which the reagent is decolorized. 3. Note the formation of a brown suspension. Decolorization of the reagent is immediate if it occurs within 1 minute. The reaction is important because it doesn’t work on alkanes (compounds with carbon-carbon single bonds) or aromatic compounds. A negative reaction for this test indicates that the sample is either an alkane or an aromatic compound. Potassium permanganate (KMnO4) is the oxidizing agent. Permanganate converts cyclohexene into a diol. Since a syn-hydroxylation takes place, the reaction is thought to involve the formation of an intermediate cyclic manganate ester, which is readily hydrolyzed under the reaction conditions to yield the glycol. In the course of the reaction, purple permanganate is reduced to brown manganese dioxide (MnO 2). Other easily oxidized compounds such as aldehydes, some alcohols, phenols, and aromatic amines should be accounted for analysis. This reaction is used as a qualitative test for the presence of an alkene (compounds with carbon-carbon double bonds) or an alkyne (compounds with carboncarbon triple bonds). The experimental result of this test was consistent with the positive result of Baeyer’s test which is the formation of a brown suspension or the decolorization of the reagent. This indicates the presence of alkenes in flavonoids contained in the corn extract. In the case of flavonoids specifically quercetin, though it is an aromatic compound, it still contains alkenes separated from the aromatic part which caused the positive reaction for this test. Test for Aromaticity: Nitration 1. Place 2 mL of conc. HNO3 in an Erlenmeyer flask. Immerse the flask in a water bath and gradually add 2 ml conc. H2SO4. Cool the resulting mixture to room temp. This will serve as the nitrating mixture. 2. Place 5 gtts. of the sample in a dry test tube. Add 8 gtts. of the nitrating mixture and shake the test tube to ensure complete mixing. Note the formation of a yellow oily layer or droplet. Dilute with 20 gtts of water. The presence of a yellow oily layer or oil droplets indicates aromaticity of the sample. In this case, there is the occurence of the positive result which proves that the constituent extracted is aromatic in its structure. Benzene undergoes electrophilic aromatic substitution or SE in which an electrophile substitutes for one of the hydrogens attached to the benzene ring. It has a 2- step mechanism: first, benzene reacts with an electrophile (nitronium ion) forming a carbocation intermediate, second, a base (H2O) in reaction mixture pulls off a proton from the carbocation intermediate forming the more stable nitrobenzene. The first step is slow that it consumes energy while energy is released in the second fast step. Nitration Chemical Reaction Bial’s Orcinol Test Bial’s reagent: a solution of orcinol, HCl and ferric chloride 1. Two ml of a sample solution is placed in a test tube. 2. Two ml of Bial's reagent was added to the extract. 3. The solution was then heated gently in a hot water bath for 2 minutes. If the color is not obvious, more water can be added to the tube. 4. The formation of a blue-green solution indicates the presence of pentoses or nucleotides containing pentoses while the formation of a yellow-green precipitate indicates the presence of hexoses and disaccharides. Bial’s orcinol test uses concentrated hydrochloric acid (HCl) as the dehydrating acid and orcinol with a trace of ferric chloride (FeCl3) as the condensation reagent. Bial’s orcinol test is used to distinguish between pentoses and hexoses. Pentose sugar forms furfural derivatives (dehydration forming furfural) and hexose sugar forms 5hydroxymethylfurfural (dehydration forming 5-hydroxymethylfurfural) when it is heated with concentrated HCl. It is then reacted with orcinol (condensation with orcinol) in the presence of ferric ion (complexation with Fe+3) which gives a characteristic bluegreen/moss green solution for pentose sugar and yellow-green precipitate/muddy brown-gray condensation product for hexose sugar. The experimental result of this test, a yellow-green precipitate, proves that the specific constituent found in the plant has a hexose sugar in its structure. For pentose: pentose → dehydration product: → blue-green/moss green solution furfural For hexose: hexose → dehydration product: → yellow-green precipitate/ 5-hydroxymethylfurfural muddy brown-gray condensation product