ORGANIC CHEMISTRY

CHEMISTRY TERM 2
OBJECTIVES: Organic 1, Organic 2, Nuclear chemistry and Acids aaaaaaaaa7 Bases
Acids and Bases
1.
Identify acids and bases according to Arrhenius and Bronsted-Lowry.
2.
Name and write formulas for acids and bases.
3.
Explain indicators and their use.
4.
Explain and solve titration problems.
5.
Solve parent acids and parent bases of salts.
6.
Explain and solve titration.
7.
Solve Ka problems.
Words: monoprotic, polyprotic, amphoteric, hydronium ion, hydroxide ion, litmus, phenolphthalein and universal indicator.
Nuclear
1.
Define nuclear chemistry.
2.
Identify and explain the particles and rays released during radioactive decay.
3.
State characteristics of a radioactive substance.
4.
Know the proper use of a Geiger counter.
5.
Identify, complete and balance nuclear reactions.
6.
Explain basic uses of radioactive substances.
7.
Explain half-life and solve half-life problems of radioactive substances.
Organic Part 1
1.
Define organic chemistry.
2.
State the characteristics of an organic substance.
3.
Identify the four organic groups of compounds.
4.
Name the series of aliphatics and give their general formulas.
5.
Explain the hybridization of carbon.
6.
Identify and draw isotopes.
7.
Name organic compounds and draw structures of organic compounds.
Organic Part 2
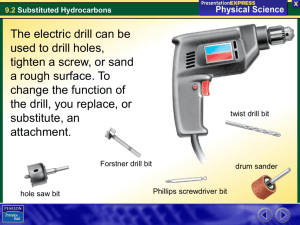
1. Identify groups of substituted hydrocarbons. (R-OH = alcohols, etc)
2. Name and draw substituted hydrocarbons.
3. Determine formulas, structures and names to substituted hydrocarbons from general information.
4. Use the Merck index to determine compound information.
You are responsible for the correct terminology in these branches of chemistry.