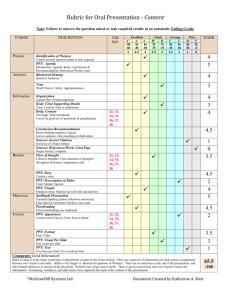
ii. special notes
advertisement

QUALITATIVE ANALYSIS OF CATIONS I. INTRODUCTION The detection and identification of ions in solution is called "Qualitative Analysis". This analysis process is based on the differing solubilities of specific groups of ions when combined with particular anions. The analysis process relies on the formation of distinctive and characteristic precipitates and colours in solution. 2 In this experiment you will be asked to analyze a solution containing one or more of the ions: Ag+, Hg 2 , Fe3+, Al3+, Zn2+ and Ni2+. In order to correctly identify the ions present in your solution, it is necessary that you work EXTREMELY carefully, making sure that you FOLLOW INSTRUCTIONS EXACTLY and that you don't mix up, contaminate or spill test tubes. It is very important that your equipment be CLEAN (but not necessarily DRY) so as to avoid accidental contamination and false results. II. SPECIAL NOTES 1. The following terms and abbreviations will be used in this lab: tt = test tube, d = drop, ppt = precipitate 2. As a rule of thumb, 1 mL = 1/8 of a test tube volume. See outline at side of page 2 for a more exact volume; you can hold the test tube up to the paper to make sure that you have 1 mL, for example. 3. Use the dropping bottles provided when asked to add a certain number of drops of a solution; don't use a dropping pipet. 4. After adding any chemical you must thoroughly stir the solution by either i) tapping the tube vigorously (as demonstrated by your teacher), or ii) using a CLEAN stirring rod. 5. If a procedure produces a transparent solution, no precipitate has been produced. If an obvious solid has formed or the solution has a milky appearance, a precipitate has been formed. "Gelatinous" precipitates have an almost–clear, flaky appearance to the solid particles; the centrifuged precipitate will have a translucent appearance (i.e. some light can pass through the precipitate). 6. When using a centrifuge, you MUST counterbalance your test tube with a second test tube filled with water to roughly the same depth as your first test tube. The second test tube is placed opposite the first one. When centrifuging a solution, leave the centrifuge "ON" for 30 s after the centrifuge has reached top speed. (At top speed the sound emitted by the centrifuge reaches a steady pitch.) 7. When using a micropipet to remove a solution from a ppt, tip the test tube as shown below. Keep the pipet tip just below the surface of the liquid and slowly remove the liquid. This will help to avoid "stirring up" the ppt. It isn't necessary to save all the liquid; as long as you get at least 90% of the liquid, uncontaminated by ppt, you will have enough to continue with subsequent tests. Once you have at least 90% of the liquid saved, simply remove the last bit of liquid and discard it without worrying about "sucking up" and discarding small bits of ppt. 8. When you are asked to "wash" a precipitate, separate off the liquid present by means of a pipet, then add the liquid to be used to wash the precipitate, stir the precipitate, centrifuge the mixture to force the precipitate to the bottom, and draw off and discard the wash solution, leaving the precipitate behind. –2– 9. If a metal ion is not completely removed from a mixture in some step, it is possible that the “left over” ions can form a bit of unwanted precipitate at a later procedure step. Hence, if you only see a few specks of precipitate in some step you can safely ignore them. Such contamination can also cause white precipitates to have a slightly grey or tan colour, and is normal. However, if a precipitate’s colour is very different from the expected colour, the metal ion you are looking for should be considered to be absent. 8 10. TESTING A SOLUTION WITH LITMUS: Tear a piece of neutral litmus into small squares and arrange on a watch glass. Take a stirring rod out of the solution to be tested and touch the rod to a litmus square. If the litmus turns PINK, the solution is ACIDIC. If the litmus turns PURPLE-BLUE, the solution is BASIC. 11. TESTING A SOLUTION WITH UNIVERSAL INDICATOR PAPER: Use the same procedure as when testing with LITMUS paper, but the colours to watch out for are: Strongly acidic = Weakly acidic = Neutral Weakly basic = Strongly basic = 4 red-pink to orange-pink orange to yellow3 = green blue-green to grey-blue 2 purple 1 12. Use a 400 mL beaker to hold “dirty” (used) stirring rods, pipets and test tubes so as to avoid getting your work place contaminated. 13. Use a grease pencil to label test tubes you are setting aside for later tests. Label the test tube with the NUMBER OF THE NEXT STEP TO BE PERFORMED ON THE CONTENTS. Note: the outside of the test tube must be wiped dry and clean of finger oil with a paper towel in order to allow the grease pencil to write properly on glass. III. PROCEDURE OBTAIN AN UNKNOWN SOLUTION AND RECORD THE SAMPLE NUMBER. Set up your lab report sheet as shown below, and record your results as you perform the procedures. You MUST accurately record the COLOUR of all precipitates and all solutions at every step, INCLUDING THE STARTING COLOUR. The marking scheme for this lab will be: Finding 6 ions to be present or absent (2 marks each) = 12 marks Quality of observations = 3 marks ————————————————————————————— Maximum mark for lab = 15 marks (Penalty for NOT submitting your sample number Procedure SAMPLE # = ... 1. Conclusion White ppt Ag+ or Hg 2 present SAMPLE COLOUR = ... Added HCl 2, 3. Centrifuged and washed ppt 4. Observations Added 10 d 5 M NH3 & heated 2 — Ppt dissolves –3– 2 Hg 2 absent = 12 marks) 2 A. Separation of the Silver Group: Ag+ and Hg 2 1. To 1 mL of your unknown solution add 5 d of 5 M HCl. Stir. 2 If a white ppt forms, one or more of Ag+ and Hg 2 may be present and you must go on to step 2. The ppt is AgCl and/or Hg2Cl2. 2 If no precipitate forms, Ag+ and Hg 2 are both absent, so that you can immediately skip to step 6 to test for the ions in Part B. 2. Centrifuge the solution from step 1. Use a pipet to remove the liquid and save it for step 6 to test for the ions in Part B. (If a few flecks of ppt float on the surface of the liquid, put the tip of the pipet below the surface of the liquid to draw up the liquid and avoid any contamination by any persistently–floating particles.) 3. Wash the ppt from step 2 by adding 1 mL of distilled water, stirring and centrifuging the mixture. Use a pipet to draw off and discard the wash liquid. 4. Add 10 d of 5 M NH3 to the ppt, heat in a boiling–water bath for 2 min with STIRRING (even if the ppt turns black immediately, this heating is necessary to complete the reaction). Centrifuge the mixture. Use a pipet to remove the liquid and test it in step 5. If the ppt is a tightly–collected black or dark–grey ppt which sits solidly on the bottom 2 of the tt, you have confirmed the presence of Hg 2 . Use a pipet to remove the liquid and test it in step 5. The ppt can be discarded. If you find that there is • no ppt, or • a dark brown ppt which floats around, OR • only a few flecks of black material are present, OR • the ppt is not black and much of the ppt dissolves. 2 then Hg 2 Is absent. Use a pipet to remove the liquid and test it in step 5. The ppt can be discarded. 5. To the solution remaining from step 4, add 1 d of phenolphthalein (the solution should be pink at this point) and then add 5 M HCl drop by drop, with stirring, until the solution loses its pink coloration (becomes acidic). A considerable amount of curdy white ppt (AgCl) at this point confirms the presence of Ag+ (a few specks should not be considered to be a positive test). Regardless of the outcome, discard the contents of the tt and proceed to step 6 to test for the ions in Part B. B. Separation of the Iron Group: Fe3+ and Al3+ 6. To the solution set aside from step 1 (or 2), add 6 d of 20 % NH4Cl, then add 5 M NH3 drop by drop, with stirring, until the solution becomes basic. (Test with litmus after each 2–3 drops; you may need several drops. Gelatinous flakes of ppt or a transparent, thin "slush" may form as the solution is becoming basic.) Then add another 10 d of 5 M NH3. STIR VERY WELL, TOP TO BOTTOM, and heat for 4 min in a boiling water bath. Centrifuge. If a gelatinous layer forms on the bottom of the tt, Fe3+ may be present as orange–brown Fe(OH)3 and/or Al3+ as white to colourless Al(OH)3. Go to step 7 to test for Fe3+ and Al3+. If NO gelatinous layer forms on the bottom of the tt, both Fe3+ and Al3+ are absent, so that you can skip to step 12 to test the solution obtained in step 6 for the ions in Part C. 7. Use a pipet to remove and save the liquid for step 12 to test for ions in Part C. Wash the ppt with 1 mL of 2 % NH4NO3. Stir, re–centrifuge, and discard the wash liquid. –4– 8. Add 12 d of 5 M NaOH and 1 mL of 3 % H2O2 to the ppt and stir. Heat in a boiling water bath for 8 minutes while stirring occasionally, leaving the stirring rod in the tt between stirring. (CARE: The solution may foam up. If it does, remove the tt from the heat and stir before returning the tt to the water bath.) At the end of this time no more tiny bubbles should be forming in the solution. If a red, brown or black ppt is present, Fe3+ may be present. Centrifuge, remove the liquid with a pipet and save it for step 11 to test for Al3+. (If the ppt is any other colour, Fe3+ is absent and you should skip to step 11 to test for Al3+.) If Fe3+ may be present, go to step 9 to test the ppt for Fe3+. If no ppt is present, Fe3+ is absent. Skip to step 11 to test this solution for Al3+. 9. To the ppt from step 8 add 1 mL of 5 M HNO3 and 4 d of 3 % H2O2, and put into a boiling water bath for 5 minutes (or less, if the ppt dissolves sooner). If the ppt dissolves, proceed to step 10. If the ppt won't dissolve, continue to boil gently for another 4 minutes, centrifuge the mixture, remove the solution and proceed to step 10. Any ppt left can be discarded. 10. You will perform TWO tests for Fe3+ on the solution set aside from step 9. If EITHER of the steps is found to be positive, you can claim Fe3+ to be present and don't need to do the other test. i) Put 1 d of the solution from step 9 onto a clean white spot plate and add 1 d of 0.1 M KSCN. A blood–red solution confirms the presence of Fe3+. ii) To the remainder of the solution from step 9, add 1 d of 0.125 M K4[Fe(CN)6], and stir. A DARK BLUE, almost opaque, solution confirms the presence of Fe3+. If the solution is any other colour, including GREEN, Fe3+ is ABSENT! You can discard this solution. 11. To the liquid from step 8, add 5 M HCl dropwise until the solution is acidic (check after each drop with a piece of universal indicator paper torn into small squares, obtainable from your teacher), then add 5 M NH3 until the solution is basic to litmus, and finally add an extra 5 d of 5 M NH3. Centrifuge the mixture. The presence of a white or colourless gelatinous layer at the bottom of the tt confirms the presence of Al3+. (If ONLY a dark ppt is present, some contaminant is present and Al3+ is absent.) Discard this mixture and proceed to step 12. C. Separation of the Zinc Group: Zn2+ and Ni2+ 12. To the solution from step 6 (or 7) add 10 d of 5 M NH3 and 10 d of saturated thioacetamide. Heat for 7 min in a water bath in a fume hood (Stench! Poisonous fumes!), then take out the test tube and allow to cool in the fume hood for a minute. If a ppt is present, one or both of Zn2+ (white ppt of ZnS) and Ni2+ (black ppt of NiS) may be present: centrifuge, discard the solution and proceed to step 13 to test the precipitate. If no ppt is present, Zn2+ and Ni2+ are both absent and you have now finished the analysis. 13. Wash the ppt from step 12 with 1 mL of 1 % NH4Cl, centrifuge and discard the wash liquid. In the fumehood, add 1 mL of distilled water and 12 d of 5 M HCl, and stir the ppt for 1 min. If all the ppt dissolves, Ni2+ is absent; go immediately to step 15 to test for Zn2+. If a ppt is present, centrifuge for 2 min, save the liquid for step 15 and proceed to step 14 to test the ppt. The presence of Ni2+ is strongly suggested if the ppt is black. –5– 14. To the ppt from step 13, add 10 d of 5 M HCl and 5 d of 5 % NaOCl. In a fume hood, heat the solution in a boiling water bath and stir until the ppt dissolves. [If the ppt doesn't dissolve completely in 3–4 min, add another 3 d of 5 % NaOCl, leave the stirring rod in the tt and boil gently for a further 3 min. Pour off the solution and discard any ppt; a sufficient amount of the ppt should have dissolved to enable you to test the solution.] Next, add 5 M NH3 until the solution is just basic (test with litmus paper). Then add about 10 d of dimethylglyoxime solution and stir. Finally, add 5 d of 5 M NH3, WITHOUT STIRRING. A fluffy pink– red ppt confirms the presence of Ni2+. [If the test turns out negative BUT you notice that on adding the NH3 a pink ppt INITIALLY formed but quickly disappeared, add several more drops of NH3 to see if you now get a positive test.] Discard this solution and proceed to step 15. 15. Put the solution from step 13 onto a clean evaporating dish and heat almost to dryness (that is, until only a few drops of liquid remain.) with a bunsen burner. [Note: it's OK if all the liquid does evaporate, just don't "bake" the solution to dryness.] Add 1 mL distilled water, stir well to dissolve any solid present, and transfer the resulting solution to a small test tube with a dropper. This solution will be tested for the presence of Zn2+ using the "Zinc Test Kit" at the back of the room. (IMPORTANT: all equipment used in this test must be very clean – the test is so sensitive that it can detect minute amounts of zinc ion contaminants from a previous user of the equipment.) Using a clean pipet and a clean, dry, white spot plate, add 2 drops of the solution to be tested to one depression of the spot plate. To the solution in the depression add 1 d of 1 M H2SO4, followed by 1 d of 0.02 % CoSO4, and finally 2 d of ammonium mercuri–thiocyanate reagent. Use a clean stirring rod to gently stir the mixture in the spot plate depression, then let the mixture settle. • If a sky–blue ppt is seen within 15 seconds (as a granular spot about 0.5 cm across at the bottom of the depression) Zn2+ is present. • If the solution remains colourless, or if the ppt is any other colour than blue, Zn2+ is absent. IV. CONCLUSION Submit your experimental notes giving the details of the tests you performed, the observations you made, and the conclusions you made. A set of scrawled comments on the hand–out procedure sheets is not acceptable. At the end of the sheets, summarize your results by stating the ions you found, your sample number and the initial colour of the solution you were given. –6– FLOW CHARTS FOR SEPARATION STEPS 2 Part A: Separation of the Silver Group: Ag+and Hg 2 –7– Part B: Separation of the Iron Group: Fe3+, Al3+ –8– Part C: Separation of the Zinc Group: Zn2+, Ni2+