Do Now - Barren County Schools
advertisement
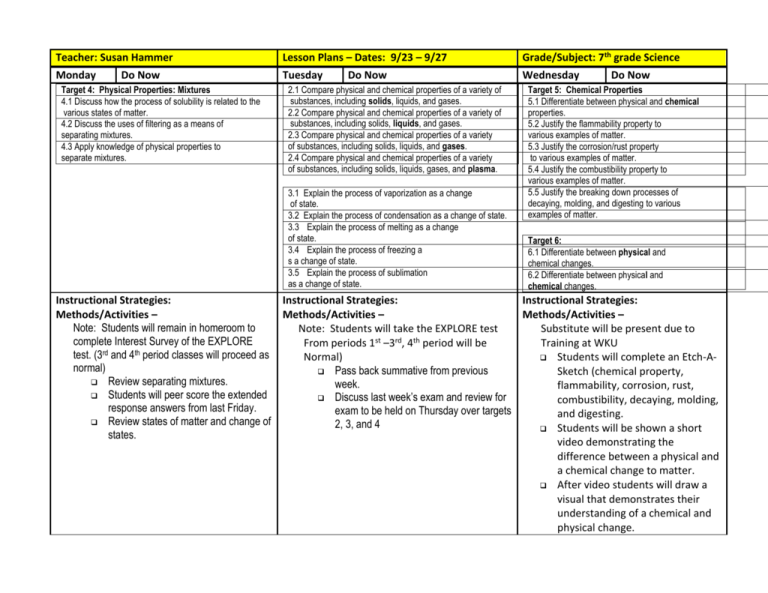
Teacher: Susan Hammer Lesson Plans – Dates: 9/23 – 9/27 Grade/Subject: 7th grade Science Monday Tuesday Wednesday Do Now Target 4: Physical Properties: Mixtures 4.1 Discuss how the process of solubility is related to the various states of matter. 4.2 Discuss the uses of filtering as a means of separating mixtures. 4.3 Apply knowledge of physical properties to separate mixtures. Do Now 2.1 Compare physical and chemical properties of a variety of substances, including solids, liquids, and gases. 2.2 Compare physical and chemical properties of a variety of substances, including solids, liquids, and gases. 2.3 Compare physical and chemical properties of a variety of substances, including solids, liquids, and gases. 2.4 Compare physical and chemical properties of a variety of substances, including solids, liquids, gases, and plasma. 3.1 Explain the process of vaporization as a change of state. 3.2 Explain the process of condensation as a change of state. 3.3 Explain the process of melting as a change of state. 3.4 Explain the process of freezing a s a change of state. 3.5 Explain the process of sublimation as a change of state. Instructional Strategies: Methods/Activities – Note: Students will remain in homeroom to complete Interest Survey of the EXPLORE test. (3rd and 4th period classes will proceed as normal) Review separating mixtures. Students will peer score the extended response answers from last Friday. Review states of matter and change of states. Do Now Target 5: Chemical Properties 5.1 Differentiate between physical and chemical properties. 5.2 Justify the flammability property to various examples of matter. 5.3 Justify the corrosion/rust property to various examples of matter. 5.4 Justify the combustibility property to various examples of matter. 5.5 Justify the breaking down processes of decaying, molding, and digesting to various examples of matter. Target 6: 6.1 Differentiate between physical and chemical changes. 6.2 Differentiate between physical and chemical changes. Instructional Strategies: Instructional Strategies: Methods/Activities – Methods/Activities – Note: Students will take the EXPLORE test Substitute will be present due to st rd th From periods 1 –3 , 4 period will be Training at WKU Normal) Students will complete an Etch-A Pass back summative from previous Sketch (chemical property, week. flammability, corrosion, rust, Discuss last week’s exam and review for combustibility, decaying, molding, exam to be held on Thursday over targets and digesting. 2, 3, and 4 Students will be shown a short video demonstrating the difference between a physical and a chemical change to matter. After video students will draw a visual that demonstrates their understanding of a chemical and physical change. Evaluation: Extended response peer score Evaluation: Exit slip Thursday Friday Do Now Evaluation: Changes to matter drawing Do Now Targets 2, 3, and 4 (shown above) Instructional Strategies: Methods/Activities – Students will complete a test over targets 2, 3, and 4 Instructional Strategies: Methods/Activities – Students will be given back their exams and will be required to do a test analysis. After test analysis, students will finish their assignment from the day before relating to chemical and physical changes to matter etch-a-sketch and drawings. Evaluation: Exam Evaluation: Test analysis Chemical and Physical changes drawing Additional Information:






