Experiment 13
advertisement

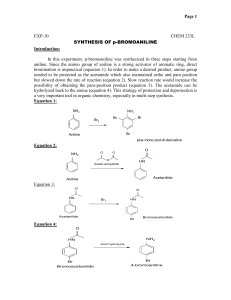
EXPERIMENT 13 ANILINE and ACETANILIDE REFERENCE: Journal of Chemical Education, pp. 488, 1979 OBJECTIVES: The student should be able to: 1. 2. 3. 4. Reduce nitrobenzene to aniline Isolate aniline and then acetanilide from the reaction mixture Calculate the amounts of reactants needed for each step in the multistep synthesis Identify the intermediate product by its melting point INTRODUCTION: These are the first and second steps in a multi-step synthesis in which you will convert nitrobenzene to pbromoaniline. Nitrobenzene, aniline and acetic anhydride are toxic compounds. Avoid contact with the liquid or inhalation of the vapors. These two steps should be considered as one experiment in lab book entries. The flow sheets for isolation of the products are to be provided by the student. REACTION EQUATIONS: 1. Reduction of Nitrobenzene to Aniline O- HCl N+ 3 Fe H2O 3FeO O NH2 Aniline Nitrobenzene 2. Acetylation O NH2 Aniline O O O N H Acetic Anhydride HO Acetic acid N-Phenyl-acetamide Acetanilide Na+ - O O Sodium Acetate REAGENTS: 1. Reactants: Iron, nitrobenzene O Solvent: tap water, 20 ml Catalyst: conc. hydrochloric acid, 2 ml 2. Reactant: Acetic anhydride Base salt: sodium acetate, 4 gm Calculate the amounts of these reactants required to yield 10.5 gms of acetanilide assuming a 100% yield. 1. Use this theoretical amount of nitrobenzene. Use a 53% excess of iron. 2. Use a 63% excess of acetic anhydride. When calculating amounts of reactants, round mole values to 3 decimal places, final masses to 1 decimal place, and volumes to whole numbers. PROCEDURE: 1. Aniline Place the iron powder and tap water in a 200 ml round bottom flask connected to a water cooled condenser set for reflux. Heat the flask with a heating mantle to boiling, turn off the heat, drop the heating mantle, and cool for two minutes. After cooling, add the concentrated hydrochloric acid through the top of the condenser. After the vigorous reaction subsides, the nitrobenzene is added in 2 ml portions over a period of about 15 minutes. Shake vigorously every 2 minutes1. After the first nitrobenzene addition, heat the system so that a gentle reflux is obtained before additional portions of nitrobenzene are added. Shake vigorously every 2 minutes and maintain a gentle reflux during nitrobenzene additions2. After the last portion of nitrobenzene has been added, most of the reaction is over and the boiling will stop if the reaction mixture is not heated. It should now be heated to boiling for an additional forty minutes and shaken frequently to keep the iron suspended. Cool the reaction mixture to near room temperature, add 20 ml of tap water, and filter using a Buchner funnel, two pieces of filter paper and gentle suction. Place the filtrate in a 500 ml erlenmeyer flask. Stir the filter cake with another 20 ml of tap water in a beaker and filter again via suction. Combine the filtrates and place the filter cake in the waste iron jar. Rinse the filter flask with 10 ml of tap water to remove the aniline which clings to the sides, and add the rinse to the combined filtrates. 2. Acetanilide Add sodium acetate to the filtrate mixture in the erlenmeyer flask and stir until it dissolves. Add an equal volume of ice and stir until the solution has cooled to 0-5C. Now add the acetic anhydride and stir the solution3. Within a few seconds, acetanilide will crystallize out of solution. Stir the solution for 5 minutes to allow the reaction to go to completion. As soon as the ice has melted filter the acetanilide crystals through a Buchner funnel with suction. Apply suction for an extra minute or two so that the filter cake will be as dry as possible4. The crude acetanilide is saved for use in Experiment 14, the continuation of our multi-step synthesis.5 NOTES Note 1 The whole ringstand assembly can be shaken so as to keep the iron powder suspended in the liquid. Note 2 It is extremely important that the mixture be shaken vigorously after each addition and heated if necessary to maintain gentle boiling. Note 3. Acetic anhydride is highly corrosive to biological material and is also a lacramator. If this reagent comes in contact with the skin, it must be washed off immediately with a large quantity of water. This portion of the procedure must be done under the hood. Use a dry graduated cylinder to measure the acetic anhydride. Note 4. To be certain of the identity of the product, a portion should always be purified even if the crude product is satisfactory for its intended use. Here a small portion of the acetanilide should be purified by recrystallization from water with the use of decolorizing charcoal if necessary. The melting point of the purified material is run. Note 5 The crude acetanilde obtained in this experiment maybe used immediately, without drying, in Experiment 13 in order to complete the experiments in a timely manner. TPS REV 5/7/03