!"#$%&'(()*+,,-./#"0123"#4% ,% 560('$*7*%"8%.3$(.07-79$%8:"#%.07-70$
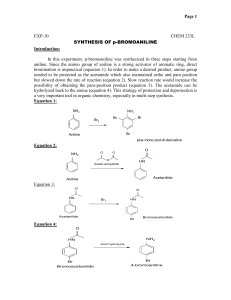
Synthesis of acetanilide from aniline
;$97370.<'$#7*(:6
Synthesis of Acetanilide from Aniline l Medicinal Chemistry l Labmonk
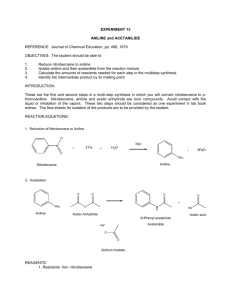
BACKGROUND
Principle:
Acetanilide is synthesized from aniline (https://www.amazon.in/gp/product/1356938906
ie=UTF8&tag=satyaranjan0421&creative=24630&linkCode=as2&creativeASIN=1356938906&linkId=8c43e9cadbb095f4fa04e77
acetylating it with acetic anhydride in presence of glacial acetic acid. Aniline or phenylamine is a pri
nature. Acetic anhydride, an anhydride of acetic acid, acts as a source of acyl group here . An
anhydride to form Acetanilide by nucleophilic substitution reaction and the reaction is called acet
aniline acts as the nuclepohile and acyl (CH3CO-) group from acetic anhydride acts as the electrop
atom of –NH2 group is replaced by the acyl group.1
Aim: To prepare acetanilide from aniline.
Reaction:
Mechanism:
Use:
It is an antipyretic agent.
REQUIREMENTS
Chemicals:
Acetic acid/anhydride mixture – 20 ml
Aniline – 10 ml
Apparatus:
Conical flask – 250 ml
Reflux water-condenser set
Buchner funnel
Measuring cylinder
Filter paper
PROCEDURE
Add 20 ml of a mixture of acetic anhydride (https://labmonk.com/synthesis-of-p-bromoacetanilid
glacial acetic acid (equal volumes) to 10 ml (10.3 g) of aniline (https://labmonk.com/to-prepare-and-s
aniline) in a conical flask of 250 ml. Fit a reflux water-condenser to the flask and gently boil the m
pour the hot liquid into 200 ml of cold water with constant stirring. The acetanilide (https://labm
submit-acetanilide-from-aniline) quickly crystallises. Filter yield by a pump and wash the crude ace
Recrystallise from about 60 ml of a mixture of one volume of acetic acid (https://labmonk.com/synt
acid-from-salicylic-acid) and two volumes of water: filter off the colourless crystals at the pump, aga
water, drain and dry.
Note: Alternatively, the crude acetanilide may be recrystallised from boiling water, but in this case
(about 300 ml) of the solvent will be required.
Calculation:
Here limiting reagent is aniline; hence yield should be calculated from its amount taken.
Molecular formula of aniline = C6H7N1
Molecular formula of acetanilide = C8H9O1N1
Molecular weight of aniline = 93 g/mole
Molecular weight of acetanilide = 135 g/mole
Theoretical yield:
1.g aniline forms 135 g acetanilide
Therefore, 10.3 g (10 ml) aniline will form ………? (X) g acetanilide
X =( 135 × 10.3)/93 = 14.95 g
Theoretical yield = 14.95 g
Practical yield = ————- g
% Yield = (Practical Yield)/(Theoretical Yield) × 100
CONCLUSION
=3$(.07-79$%>.*%*60('$*7?$9%.09%('$%)$:3$0(.@$%&'(()*+,,-./#"0123"#,.0.-@$*73A.3(7B7(6A*(C96A"8A9:C@*A/6A(
8"C09%("%/$EEE22F%&;2)2%GGHIJ%67$-9K%GL%@242
REFERENCES
G2% M:.3(73.-%
N:@.073%
<'$#7*(:6%
7$SWXYPZ(.@S*.(6.:.0[.0LRA
&'(()*+,,>>>2.#.?"0270,@),):"9C3(,LOPQRRQQPG
QGZ3:$.(7B$SQR]HLZ-701<"9$S.*QZ3:$.(7B$=5^_SLOPQRRQQPGZ-701^9SO.QP$$O$.P9H`3
/6%Y:$9$:731%b$":@$%;.00%.09%c$:0.:9%<'.:-$*%5.C09$:*%MC/-7*'$9%/6%d"0@.0%^032K%Y"
GLP2
\97(":7.-%X$.#
Ultra High Purity C60 99.99+%
Sublimed (no Chemicals) C60
MTR - Mass Production of Sublimed HighPurified C60 for Supplement Industry,
Cosmetics etc
mtr-ltd.com
OPEN
5!=f\%X!^5
! !"%#$%&'
" %&'(()+,,>>>28.3$/""123"#,*'.:$:2)')VCS'(()*+,,-./#"0123"#,*60('$*7
8:"#A.07-70$4
#%&'(()*+,,(>7(($:23"#,*'.:$VC:-S'(()*+,,-./#"0123"#,*60('$*7*A"8A.3$(.07$%&'(()*+,,)-C*2@""@-$23"#,*'.:$VC:-S'(()*+,,-./#"0123"#,*60('$*7*A"8A.3
.07-70$4
%%&'(()*+,,>>>2-701$97023"#,*'.:$=:(73-$VC:-S'(()*+,,-./#"0123"#,*60('$*
8:"#A.07-70$4
&%&>'.(*.))+,,*$09V($e(S'(()*+,,-./#"0123"#,*60('$*7*A"8A.3$(.07-79$A
'%&#.7-("+V*C/[$3(S560('$*7*%"8%.3$(.07-79$%8:"#
.07-70$Z/"96S'(()*+,,-./#"0123"#,*60('$*7*A"8A.3$(.07-79$A8:"#A
f\d=X\g%=fX^<d\5
(
&'(()*+,,-./#"0123"#,*60('$*7*A"8A).:.3$(.#"-A8:"#A)A
.#70")'$0"-4
Synthesis of paracetamol from p-aminophenol
(https://labmonk.com/synthesis-of-paracetamolfrom-p-aminophenol)