Presentation 6b - Table Information
advertisement

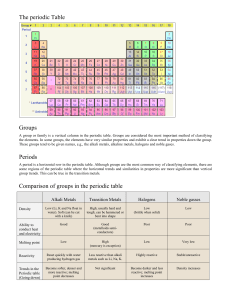
The Periodic Table of the Elements The table contains a stair division on the right side. The elements to the left of this line are METALS (except Hydrogen). The elements to the right of the line are the NONMETALS. Many of these are gases. The solids, such as carbon are very brittle and easily crushed. The properties of the elements change gradually as they move from left to right. On either side of the stair step line are the metalloids. Elemental Families. Elements found in the same column of the periodic table are said to belong to the same FAMILY or GROUP. Elements are grouped into the same families because they have similar properties. Some Important Families. Family 1 The Alkali Metals These are very soft metals. They all contain one electron on their outer energy level. This makes them highly reactive and must be stored under oil to prevent a reaction with the air. They are NEVER found uncombined in nature. They will react immediately with air, water, etc. The most reactive of these would be francium because its' outer level is the farthest away from the pull of the nucleus. Alkali metals and the compounds that contain them are widely used. The salt used to season food and the rock salt used on roads contain sodium. Sodium also plays a large part in the production of glass. Family 2 The Alkaline Earth Metals These metals are not quite as reactive as the Alkali metals but they are also not found uncombined in nature. The compounds that they form are found in powders, plasters and cements and are widely used in industry. When an Alkaline earth metal dissolves in the ground water it produces hard water. Hard water contains many dissolved minerals essential for good health however it also creates many problems for water works companies because it will deposit on pipes and equipment. Family 17 The Halogens These elements are called the salt forming family. "Halogen" comes from the Greek word meaning "Salt-makers". They only require one electron to complete their outer energy level and become inert. Fluorine is the most reactive of these non-metals because it's outer level is very close to the nucleus and the attraction is great. The uses of Halogens and their compounds are wide spread. A chlorine based compound is used in pools and disinfectant bleaches. Fluorine compounds are used in dental health. Teflon is a fluorine compound that forms non-stick coating for cookware and skis. Family 18 The Nobel Gases These elements were once thought to be absolutely unreactive, however they can be forced to react under extreme conditions. They do not react because their outer most lever already contains 8 electrons. Helium is used to fill airships and weather balloons. Light bulbs and some newly produced windows contain argon gas. Neon gas will produce a bright light when an electrical current is passed through it. The Transition Elements These elements are found in families 3-12. The are sometimes referred to as the "heavy metals" because of their high densities. These elements are used either alone or as alloys to produce our most commonly used structural metals. A few transition metals, like copper can occur naturally, but most occur in compounds called ORES from which the metals can be extracted. Their electron configuration is not as clear as with the other families. They contain partially filled energy levels. Synthetic Elements You could take apart Earth, piece by piece, but you would never find even the slightest trace of an element called promethium. That's because promethium is a synthetic element, one that is produced only in a laboratory. Like all synthetic elements promethium is radioactive. Lanthanides and Actinides Promethium belongs to a part of the periodic table known as the lanthanide series. These elements share the similar chemical properties. The same is true of the elements belonging to the actinide series. Most of the synthetic elements are found in the actinide series and are known as transuranium meaning "beyond uranium". The first transuranium element created was called neptunium because Neptune was the nest planet after Uranus. Later that year another element was discovered and named plutonium. The Plutonium Problem Plutonium is probably the best known of the synthetic elements. It has been used in nuclear warheads and on the Voyager spacecraft. Nearly 40 metric tons of plutonium are produced each year. Most of it in the form of nuclear waste. Finding was to dispose of plutonium is one of society's most challenging problems. Plutonium is one of the most toxic substances on Earth- a lump size of an orange could poison an entire city and it would remain radioactive for more than 24 000 years. Many experts believe that the safest way to dispose of Plutonium is to bury it deep underground. The United States government is considering burying the Plutonium deep inside a mountain range in Nevada. Before it is buried it must be sealed in specially designed containers. These containers have been subjected to extreme tests in order to prove their indestructibility. Despite all of these tests no one is absolutely sure they can withstand all possible forces for eternity. The people of Nevada are concerned about this possible dump site. What would happen in an earthquake, a volcano, or if they came in contact with underground water?