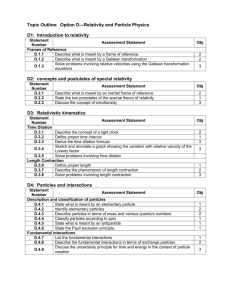
IBO Physics Topics
advertisement

C O R E T O P I C S - BOTH STANDARD AND HIGHER LEVEL 1 Physics and physical measurement 1.1 1.1.1 1.1.2 The realm of physics State and compare quantities to the nearest order of magnitude. State the ranges of magnitude of distances, masses and times that occur in the universe, from smallest to greatest. State ratios of quantities as differences of orders of magnitude. Example: The ratio of the diameter of the hydrogen atom to its nucleus is about five orders of magnitude (5 powers of 10). Estimate approximate values of everyday quantities to one or two significant figures and/or to the nearest order of magnitude. 1.1.3 1.1.4 1.2 Measurement and uncertainties The SI system of fundamental and derived units 1.2.1 State the fundamental units in the SI system. Know kilogram, meter, second, ampere, mole and kelvin. 1.2.2 Distinguish between fundamental and derived units and give examples of derived units. 1.2.3 Convert between different units of quantities. Example:.J and kWh and eV all measure energy in a different unit. 1.2.4 State units in the accepted SI format. Use ms-1 instead of m/s. 1.2.5 State values in scientific notation and in multiples of units with appropriate prefixes. Be able to use prefixes such as nano and giga. Uncertainty and error in measurement 1.2.6 Describe and give examples of random and systematic errors. Systematic errors are NOT reduced by repeated readings. 1.2.7 Distinguish between precision and accuracy. Precision is GROUPING of repeated measurements, and accuracy is how close to TARGET measurements average out to be. 1.2.8 Explain how the effects of random errors may be reduced. Random errors are ‘averaged out.’ 1.2.9 Calculate quantities and results of calculations to the appropriate number of significant figures. The number of sig. figs. reflects the precision of the least precise of the measured values. Uncertainties in calculated results 1.2.10 State uncertainties as absolute, fractional and percentage uncertainties. The symbol is read ‘the uncertainty in...’ 1.2.11 Determine the uncertainties in results. If y = a +b then y = a + b. If y = ab/c then y/y =a/a + b/b + c/c. Uncertainties in graphs 1.2.12 Identify uncertainties as error bars in graphs. 1.2.13 State random uncertainty as an uncertainty range (+/-) and represent it graphically as an ‘error bar.’ 1.2.14 Determine the uncertainties in the gradient and intercepts of a straight-line graph. 1.3 1.3.1 Vectors and scalars Distinguish between vector and scalar quantities and give examples of each. Vectors have magnitude and direction (velocity, displacement, force, acceleration). Scalars only have magnitude (time, pressure, speed, distance, mass). 1.3.2 Determine the sum or difference of two vectors by a graphical method. Multiplication and division of vectors by scalars is also required. Be sure to distribute the factor to EACH COMPONENT. 1.3.3 Resolve vectors into perpendicular components along chosen axes. Ax = A cos Ay = A sin 2 Mechanics 2.1 2.1.1 Kinematics Define displacement, velocity, speed and acceleration. NOTE: IB uses u for initial velocity, and s for displacement. Many books use vo and x for those same quantities. Explain the difference between instantaneous and average values of speed, velocity and acceleration. -The smaller t is the more ‘instantaneous’ a quantity is. Outline the conditions under which the equations for uniformly accelerated motion may be applied. -The kinematic equations s = ut + (1/2)at2, and v = u + at, and v2 = u2 + 2as can only be used if the acceleration a is CONSTANT. Identify the acceleration of a body falling in a vacuum near the Earth’s surface with the acceleration g of freefall. Solve problems involving the equations of uniformly accelerated motion. -General: s = ut + (1/2)at2, and v = u + at, and v2 = u2 + 2as, and s = (u + v)t/2; -Freefall: Substitute ‘-g’ for ‘a’ in all of the above equations. 2.1.2 2.1.3 2.1.4 2.1.5 2.1.6 2.1.7 2.1.8 2.1.9 2.2 2.2.1 2.2.2 2.2.3 2.2.4 2.2.5 2.2.6 2.2.7 2.2.8 2.2.9 2.2.10 Describe the effects of air resistance on falling objects. -Students should know what is meant by terminal speed. -This is when the drag force exactly balances the weight. Draw and analyze distance-time graphs, displacement-time graphs, velocity-time graphs and the areas under the velocity-time graphs and acceleration-time graphs. -The slope of the displacement-time graph is the velocity. -The slope of the velocity-time graph is the acceleration. -The area under the velocity-time graph is the total displacement. Calculate and interpret the gradients (slopes) of displacement-time graphs and velocity-time graphs. -The (gradient) slope of the displacement-time graph is the velocity. -The slope of the velocity-time graph is the acceleration. Determine relative velocity in one and two dimensions. vab = va - vb. This formula is NOT in the Physics Data Booklet. Forces and dynamics Calculate the weight of a body using the expression W = mg. -Weight W is just a special force. Since weight causes freefall acceleration, F = W, and a = g, so that F = ma becomes W = mg. Identify the forces acting on an object and draw free-body diagrams representing the forces acting. -Know normal, tension, friction, weight, drag, and spring forces. Determine the resultant force in different situations. F = ma, or Fnet = ma. State Newton’s first law. -An object at rest stays at rest, or an object in uniform motion remains in its state of uniform motion, unless there is a net force applied to it. Describe examples of Newton’s first law. -Any non-accelerating body, such as a plane traveling in a constant direction at a constant speed, or an object at rest. State the condition for translational equilibrium. -F = 0 (not on your Physics Data Booklet). -This could imply no motion, or constant velocity. Solve problems involving translational equilibrium. F = 0, balanced forces. -Note that F = 0 does not imply the object is standing still. State Newton’s second law of motion. F = ma, or Fnet = ma. -The momentum form looks like this: F = p/t (see 2.2.10). Solve problems involving Newton’s second law. F = ma, or Fnet = ma. Define linear momentum and impulse. p = mv, and Impulse = Ft = mv = area under F vs. t graph and Fnet = p/t. 2.2.11 2.2.12 2.2.13 2.2.14 2.2.15 2.3 2.3.1 2.3.2 2.3.3 2.3.4 2.3.5 2.3.6 2.3.7 2.3.8 Determine the impulse due to a time-varying force by interpreting a forcetime graph. -Impulse is the area under the force-time graph. State the law of conservation of linear momentum. In the absence of an external force, momentum is conserved. Po = Pf. Solve problems involving momentum and impulse. pao + pbo = paf + pbf (in general), and mavao + mbvbo = (ma + mb)vf (inelastic collision). State Newton’s third law. For every action there is an equal and opposite reaction. Fab = Fba. Discuss examples of Newton’s third law. Students should understand that when two bodies interact, the forces they exert on each other are equal and opposite. Work, energy and power Outline what is meant by work. W = Fs cos . Determine the work done by a non-constant force by interpreting a forcedisplacement graph. -The work done by a force is the area under the force-displacement graph. -For a spring, this is a triangular area. Solve problems involving the work done by a force. W = Fs cos . Don’t forget the work-kinetic energy theorem: Work = K = EK. Outline what is meant by kinetic energy. EK = (1/2)mv2. -IBO uses EK for the K used in many textbooks. -In terms of momentum, EK = p2/(2m). Outline what is meant by change in gravitational potential energy. EP = mgh. -IBO uses EP for the U used in many books for potential energy. State the principle of conservation of energy. EK + EP = 0 (as used by IBO) Ko + Uo = Kf + Uf, or K + U = 0 or K + U = Wnc. -None of these formulas is in the Physics Data Booklet. List different forms of energy and describe examples of the transformation of energy from one form to another. -Mechanical (kinetic and potential). -Chemical. -Heat. -Nuclear. Distinguish between elastic and inelastic collisions. -In elastic collisions kinetic energy is conserved. 2.3.9 2.3.10 2.3.11 2.4 2.4.1 2.4.2 2.4.3 2.4.4 Define power. P = E/t, or P = Fv. Power = Work/Time. Define and apply the concept of efficiency. Efficiency = Eout/Ein (not in Physics Data Booklet). Solve problems involving momentum, work, energy and power. Uniform circular motion Draw a vector diagram to illustrate that the acceleration of a particle moving with constant speed in a circle is directed towards the center of the circle. Apply the expression for centripetal acceleration. a = v2/r or a = 42r/T2, where T is the period of motion (time to go around once). Identify the force producing circular motion in various situations. Examples include friction of tires on turn, gravity on planet/moon, cord tension. Solve problems involving circular motion. -Problems of banked roads or banking airplane turns will not be included. Fc = mac -where Fc is the centripetal force and ac is the centripetal acceleration. 3 Thermal physics 3.1 3.1.1 Thermal concepts State that temperature determines the direction of thermal energy transfer between two objects. Heat flows from bodies with higher temperature into bodies with lower temperature until thermal equilibrium is met. State the relation between the Kelvin and Celsius scales of temperature. T(K) = T(°C) + 273 State that the internal energy of a substance is the total potential energy and random kinetic energy of the molecules of the substance. -potential energy arises from forces of interaction between molecules, and -kinetic energy is the average random/ translational/rotational motion of the molecules. Explain and distinguish between the macroscopic concepts of temperature, internal energy and thermal energy (heat). Define mole and molar mass. Molar mass is the mass of a mole of a molecule (6.02×1023 molecules) and is equal to the gram atomic weight. Define the Avogadro constant. 6.02×1023 molecules. 3.1.2 3.1.3 3.1.4 3.1.5 3.1.6 3.2 Thermal properties of matter Specific heat capacity, phase changes and latent heat 3.2.1 Define specific heat capacity and thermal capacity. 3.2.2 3.2.3 3.2.4 3.2.5 3.2.6 3.2.7 3.2.8 -The c in Q = mcT. -Be sure when you define a concept using a formula you explain each part of the formula. Solve problems involving specific heat capacities and thermal capacities. Q = mcT. Explain the physical differences between the solid, liquid, and gaseous phases in terms of molecular behavior. Describe and explain the process of phase changes in terms of molecular structure and particle motion. Explain in terms of molecular behavior why temperature does not change during a phase change. -During phase changes heat energy goes into changing the potential energy of the forces bonding the molecules, rather than changing the kinetic energies of the molecules. Distinguish between evaporation and boiling. Define specific latent heat. -The L in Q = mL. -Be sure when you define a concept using a formula you explain each part of the formula. -There is no temperature change during phase change, yet energy is added or removed to break or make inter-molecular bonds. Solve problems involving specific latent heats. Q = mL. Kinetic model of an ideal gas 3.2.9 Define pressure. p = F/A. 3.2.10 State the assumptions of the kinetic model of an ideal gas. 3.2.11 State that the temperature is a measure of the random kinetic energy of the molecules of an ideal gas. 3.2.12 Explain the macroscopic behavior of an ideal gas in terms of a molecular model. For example, how does a change in volume produce a change in pressure or temperature? -Be able to use pV = nRT. 4 Oscillations and waves 4.1 4.1.1 Kinematics and simple harmonic motion (SHM) Describe examples of oscillation. -Waves, vibrations, pendulums, masses on springs, sound, light. Define the terms displacement, amplitude, frequency, period, and phase difference. Define simple harmonic motion (SHM) and state the defining equation as a = -2x. -Know the significance of the (-) sign: the acceleration is caused by a RESTORING FORCE. 4.1.2 4.1.3 4.1.4 4.1.5 4.1.6 4.2 4.2.1 4.2.2 4.2.3 4.3 4.3.1 4.3.2 4.3.3 4.3.4 4.3.5 4.3.6 Solve problems using the defining equation for SHM. -The defining equation of SHM is a = -2x. -This formula is not part of the Physics Data Booklet. Apply the equations as solutions to the defining equation a = -2x. = 2/T -where xo and vo are the maximum displacement and maximum velocity x = xo sin t and v = vo cos t or x = xo cos t and v = -vo sin t. Solve problems, both graphically and by calculation, for acceleration, velocity and displacement during SHM. v = sqrt[2(xo2-x2)]. Energy changes during SHM Describe the interchange between kinetic energy and potential energy during SHM. EP + EK = Etotal = const -if no friction. -This formula in not in the Physics Data Booklet. Apply the expressions for kinetic, potential, and total energy of a particle in SHM. EK = (1/2) 2(xo2-x2) and EK(max) = (1/2)2xo2 and Etotal = (1/2)2xo2 and EP + EK = Etotal -This last is not in the Physics Data Booklet. Solve problems, both graphically and by calculation, involving energy changes during SHM. Forced oscillations and resonance State what is meant by damping. -Damping is a force that always opposes an oscillatory motion, decreasing its amplitude. Describe examples of damped oscillations. -Examples include shock absorbers on cars, and house door dampers. State what is meant by natural frequency of vibration and forces oscillations. -Think of the Tacoma Narrows bridge. Describe graphically the variation with forced frequency of the amplitude of vibration of an object close to its natural frequency. What is meant by resonance. Resonance occurs when an external energy source is applied to an object at close to its natural frequency. Its amplitude of vibration increases. Describe examples of resonance where it is useful, and where it is not. -Useful: Musical instruments. -Not useful, or harmful: Unwanted vibration that grows. 4.4 4.4.1 4.4.2 4.4.3 4.4.4 4.4.5 4.4.6 4.4.7 4.4.8 4.4.9 4.5 4.5.1 4.5.2 4.5.3 4.5.4 4.5.5 4.5.6 Wave characteristics Describe a wave pulse and a continuous progressive (traveling) wave. State that progressive (traveling) waves transfer energy. Describe and give examples of transverse and of longitudinal waves. -Transverse: Medium molecules vibrate perpendicular to wave travel. An example is a plucked string. -Longitudinal: Medium molecules vibrate parallel to wave direction. An example is a vibrating air column in a flute. Describe waves in two dimensions, including concepts of wavefronts and of rays. Describe the terms crest, trough, compression and rarefaction. Example. In a water wave, the crest is high point, the trough is low point. (Transverse wave). Example. In a sound wave, the compression is high pressure and rarefaction is low pressure. (Longitudinal wave). Define the terms displacement, amplitude, frequency, period, wavelength, wave speed, and intensity. Draw and explain displacement-time graphs and displacement-position graphs for transverse and longitudinal waves. Derive and apply the relationship between wave speed, wavelength and frequency. -Since v = /T and f = 1/T then v = f. State that all electromagnetic waves travel with the same speed in free space. -They all travel at the same speed c, the speed of light. Wave properties Describe the reflection and transmission of waves at the boundary between two media. -Reflection from a fixed boundary is 180° out of phase. -Reflection from moveable boundary is in phase. State and apply Snell’s law. n1/n2 = v2/v1 =sin2/sin1 Explain and discuss qualitatively the diffraction of waves at apertures and obstacles. -Diffraction occurs where the apertures are comparable to the wavelength Describe examples of diffraction. State the principle of superposition and explain what is meant by constructive and destructive interference. -Constructive: Crests of two waves coincide. -Destructive: Crest of one wave coincides with trough of another. State and apply the conditions for constructive and destructive interference in terms of path difference and phase difference. -Constructive: path difference = n. -Destructive: path difference = (n +.5 ) . 4.5.7 Apply the principle of superposition to determine the resultant of two waves. 5 Electric currents 5.1 Electric potential difference, current and resistance Electric potential difference 5.1.1 Define electric potential difference. V = kQ/r, V = kQ(1/r – 1/ro). 5.1.2 Determine the change in potential energy when a charge moves between two points at different potentials. U = qV. 5.1.3 Define the electron-volt. -The amount of kinetic energy an electron has after being accelerated in passing through a potential difference of 1 volt. 1 eV = 1.6×10-19J. 5.1.4 Solve problems involving electric potential difference. EP = qV = EK = (1/2)mv2. Electric current and resistance 5.1.5 Define electric current. I = q/t. 5.1.6 Define resistance. R = V/I. 5.1.7 Apply the resistance equation. R = L/A. 5.1.8 State Ohm’s law. -The voltage drop across an electrical component is proportional to the current passing through it. -The proportionality constant is the resistance. 5.1.9 Compare ohmic and non-ohmic behavior. -Ohmic components obey Ohm’s law. 5.1.10 Derive and apply expressions for electrical power dissipation in resistors. P = IV, or P = I2R, or P = V2/R. P = Work / time = qV/t = IV. 5.1.11 Solve problems involving potential difference, current and resistance. 5.2 5.2.1 5.2.2 5.2.3 Electric circuits Define electromotive force. Describe the concept of internal resistance. = I(R + r) Apply the equations for resistors in series and parallel. -Series: R = R1 + R2 + R3 + ... 5.2.8 -Parallel: 1/R = 1/R1 + 1/R2 + 1/R3 +... Draw circuit diagrams. Describe the use of ideal ammeters and ideal voltmeters. -Ideal ammeters have an internal resistance of zero are hooked up in series. -Ideal voltmeters have an internal resistance of infinity are hooked up in parallel. Describe a potential divider. A potential divider consists of at least two resistors connected in series with a battery, allowing different voltages to be tapped. Explain the use of sensors in potential divider circuits. -Sensors include light-dependent resistors (LDR) negative temperature coefficient (NTC) strain gauges. Solve problems involving electric circuits. 6 Fields and forces 6.1 6.1.1 Gravitational force and field State Newton’s universal law of gravitation. F = Gm1m2/r2. Define gravitational field strength. g = F/m. Determine the gravitational field due to one or more point masses. Derive an expression for gravitational field strength at the surface of a planet, assuming that all its mass is concentrated at its center. -Since F = mg and F = GMm/r2 then g = GM/r2. Solve problems involving gravitational forces and fields. -Don’t forget that satellites and planets are in circular motion due to the gravitational force. Thus F = GMm/r2 = mac = mv2/r. 5.2.4 5.2.5 5.2.6 5.2.7 6.1.2 6.1.3 6.1.4 6.1.5 6.2 6.2.1 6.2.2 6.2.3 6.2.4 6.2.5 Electric force and field State that there are two types of electric charge. -Positive and negative. State and apply the law of conservation of charge. -Electric charge can neither be created nor destroyed. Describe and explain the difference in electrical properties of conductors and insulators. -Conductors allow charges to move freely on their surface and inside, -Insulators prevent charges from moving. State Coulomb’s law. F = kq1q2/r2. Define electric field strength. 6.2.6 6.2.7 6.2.8 6.3 6.3.1 6.3.2 6.3.3 6.3.4 6.3.5 6.3.6 7 E = F/q. Determine the electric field strength due to one or more point charges. Draw the electric field patterns for different configurations. Solve problems involving electric charges, forces and fields. Magnetic force and field State that moving charges give rise to magnetic fields. Draw magnetic field patterns due to currents. Determine the direction of the force on a current-carrying conductor in a magnetic field. -Know the right-hand-rules. Determine the direction of the force on a charge moving in a magnetic field. -Know the right-hand-rules. Define the magnitude and direction of a magnetic field. Solve problems involving magnetic forces, fields and currents. F = qvB sin (charge q velocity v magnetic field B) F = BIL sin (current I wire length magnetic field B). Atomic and nuclear physics 7.1 The atom Atomic structure 7.1.1 Describe a model of the atom that features a small nucleus surrounded by electrons. 7.1.2 Outline the evidence that supports a nuclear model of the atom. -Know Rutherford’s alpha particle scattering experiments. 7.1.3 Outline one limitation of the simple model of the nuclear atom. 7.1.4 Outline evidence for the existence of atomic energy levels. -Know about the Balmer, Paschen and Lyman hydrogen spectra series. Nuclear structure 7.1.5 Explain the terms nuclide, isotope and nucleon. -Nuclide = isotope = same element with different number of neutrons. 7.1.6 Define nucleon number A, proton number Z and neutron number N. A = Z + N. 7.1.7 Describe the interactions in a nucleus. -There is a repulsive Coulomb interaction between protons countered by the strong, short-range nuclear force. 7.2 Radioactive decay Radioactivity 7.2.1 Describe the phenomenon of natural radioactive decay. -The inclusion of the antineutrino should be mentioned in the case of the beta(-) decay. This antineutrino is required to conserve momentum. 7.2.2 7.2.3 7.2.4 7.2.5 Half-life 7.2.6 7.2.7 7.2.8 7.2.9 Describe the properties of alpha and beta particles and gamma radiation. -Alpha has least penetration, then comes beta, then comes gamma. Describe the ionizing properties of alpha and beta particles and gamma radiation. Outline the biological effects of ionizing radiation. Explain why some nuclei are stable and others are unstable. An explanation regarding relative numbers of protons and neutrons is sufficient. State that radioactive decay is a random and spontaneous process and that the rate of decay decreases exponentially with time. Define the term radioactive half-life. Determine the half-life of a nuclide from a decay rate. t 1/2 = ln(2) / . Solve radioactive decay problems involving integral numbers of half-lives. 7.3 Nuclear reactions, fission and fusion Nuclear reactions 7.3.1 Describe and give an example of an artificial (induced) transmutation. 7.3.2 Construct and complete nuclear equations. -Balance A, Z and N using A = Z + N. 7.3.3 Define the term unified atomic mass unit. 7.3.4 Apply the Einstein mass-energy equivalence relationship. E = mc2. 7.3.5 Define the concepts mass defect, binding energy, and binding energy per nucleon. 7.3.6 Draw and annotate a graph showing the variation with nucleon number of the binding energy per nucleon. 7.3.7 Solve problems involving mass defect and binding energy. Fission and fusion 7.3.8 Describe the processes of nuclear fission and nuclear fusion. -Fission-splitting of nuclei. -Fusion-joining of nuclei . 7.3.9 Apply the graph in 7.3.6 to account for the energy release in the processes of fission and fusion. 7.3.10 State that nuclear fusion is the main source of the Sun’s energy. 7.3.11 Solve problems involving fission and fusion reactions. 8 Energy, power, and climate change 8.1 Energy degradation and power generation 8.1.1 8.1.2 8.1.3 8.1.4 8.2 8.2.1 8.2.2 8.2.3 8.2.4 8.2.5 8.2.6 8.3 8.3.1 8.3.2 8.3.3 8.3.4 8.3.5 State that thermal energy may be completely converted to work in a single process, but that continuous conversion of this energy into work requires a cyclical process and the transfer of energy from the system. Explain what is meant by degraded energy. Energy lost as heat to the surrounding environment during conversion from one form to another. Construct and analyze energy flow diagrams (Sankey diagrams) and identify where the energy is degraded. Outline the principal mechanisms involved in the production of electrical power. World energy sources Identify different world energy sources. -Know which ones produce CO2. Outline and distinguish between renewable and non-renewable energy sources. Define the energy density of a fuel. -Measured in J/kg. Discuss how choice of fuel is influenced by its energy density. State the relative proportions of world use of the different energy sources that are available. Oil 38%, Gas 23%, Coal 26%, Hydroelec 6%, Nuclear 6%, All others 1%. Discuss the relative advantages and disadvantages of various energy sources. Fossil fuel power production Outline the historical and geographical reasons for the widespread use of fossil fuels. Discuss the energy density of fossil fuels with respect to the demands of power stations. Discuss the relative advantages and disadvantages associated with the transportation and storage of fossil fuels. State the overall efficiency of power stations fuelled by different fossil fuels. Describe the environmental problems associated with the recovery of fossil fuels and their use in power stations. 8.4 Non-fossil fuel power production Nuclear power 8.4.1 Describe how neutrons produced in a fission reaction may be used to initiate further fission reactions (chain reactions), 8.4.2 Distinguish between controlled nuclear fission (power production) and uncontrolled nuclear fission (nuclear weapons). -Controlled: Each fission starts one more reaction. -Uncontrolled: Each fission starts more than one reaction. 8.4.3 Describe what is meant by fuel enrichment. -Centrifuge or diffusion to concentrate percentage of uranium-235. 8.4.4 8.4.5 8.4.6 8.4.7 8.4.8 8.4.9 8.4.10 8.4.11 Describe the main energy transformations that take place in a nuclear power station. Discuss the role of the moderator and the control rods in the production of controlled fission in a thermal fission reactor. Discuss the role of the heat exchanger in a fission reactor. Describe how neutron capture by a nucleus of uranium-238 results in the production of a nucleus of plutonium-239. Describe the importance of plutonium-239 as a nuclear fuel. -Know that it is used as a fuel in other types of nuclear reactors. Discuss safety issues and risks associated with the production of nuclear power. Outline problems associated with the production of nuclear power using fusion. More energy needed to produce fusion than is harvested. Solve problems on the production of nuclear power. Be able to construct and balance nuclear reaction equations. Solar power 8.4.12 Distinguish between a photovoltaic cell and a heating panel. -Photovoltaic cell produces electricity from sunlight. -Heating panel produces hot water from sunlight. 8.4.13 Outline reasons for seasonal and regional variations in the solar power incident per unit area of the Earth’s surface. 8.4.14 Solve problems involving specific applications of photovoltaic cells and solar heating panels. I = power/A. Hydroelectric power 8.4.15 Distinguish between different hydroelectric schemes. 8.4.16 Describe the main energy transformations that take place in a hydroelectric scheme. -Potential energy of water to -kinetic energy to -turbine motion to -electricity generation to -I2R loss during transmission. 8.4.17 Solve problems involving hydroelectric schemes. Wind power 8.4.18 Outline the basic features of a wind generator. 8.4.19 Determine the power that may be delivered by a wind generator, assuming that the wind kinetic energy is completely converted into mechanical energy, and explain why this is impossible. 8.4.20 Solve problems involving wind power. Power = (1/2)Av3 Wave power 8.4.21 8.4.22 8.4.23 Describe the principle of operation of an oscillating water column (OWC) ocean-wave energy converter. Determine the power per unit length of a wavefront, assuming a rectangular profile for the wave. Solve problems involving wave power. power per unit length = (1/2)A2gv. 8.5 Greenhouse effect Solar radiation 8.5.1 Calculate the intensity of the Sun’s radiation incident on a planet. Power = AT4, where A = 4πr2 for a spherical surface. 8.5.2 Define albedo. albedo = total scattered power divided by total incident power. 8.5.3 State factors that determine a planet’s albedo. -Know that the Earth’s albedo varies daily and is dependent on season, cloud formations, and latitude. -Oceans have a low value but snow has a high value. -The global annual mean albedo is 0.3 (30%) on Earth. The greenhouse effect 8.5.4 Describe the greenhouse effect. -Trapping of earth’s radiant heat (IR) by greenhouse gases in the atmosphere on the way back out. 8.5.5 Identify the main greenhouse gases and their sources. -CH4, H2O, CO2 and N2O are the main ones. -Each has natural and man-made origins. 8.5.6 Explain the molecular mechanisms by which greenhouse gases absorb infrared radiation. -Know the role played by resonance. The natural frequency of oscillation of the greenhouse gas molecules is in the infrared range. 8.5.7 Analyze absorption graphs to compare the relative effects of different greenhouse gases. 8.5.8 Outline the nature of blackbody radiation. 8.5.9 Draw and annotate a graph of the emission spectra of black bodies at different temperatures. 8.5.10 State the Stefan-Boltzmann law and apply it to compare emission rates from the different surfaces. Power = AT4. 8.5.11 Apply the concept of emissivity to compare the emission rates from the different sources. Power =eAT4. 8.5.12 Define the surface heat capacity Cs. Cs = Q /(AT). 8.5.13 Solve problems on the greenhouse effect and the heating of planets using a simple energy balance climate model. T = ( Iin - Iout) t /Cs. 8.6 8.6.1 8.6.2 8.6.3 8.6.4 8.6.5 8.6.6 8.6.7 8.6.8 8.6.9 8.6.10 8.6.11 8.6.12 Global warming Describe some possible models of global warming. State what is meant by the enhanced greenhouse effect. -That portion of the GHE caused by mankind. Identify the increases combustion of fossil fuels as the likely major cause of the enhanced greenhouse effect. -Combustion of fossil fuels produces CO2 which is a greenhouse gas. Describe evidence that links global warming to increased levels of greenhouse gases. -Think of Vostok ice core samples. Outline some of the mechanisms that may increase the rate of global warming. -Increased Greenhouse gases caused by burning fossil fuels. -Decreased albedo caused by melting ice caps. Define coefficient of volume expansion. State that one possible effect of the enhanced greenhouse effect is a rise in mean sea level. Outline possible reasons for a predicted rise in mean sea level. -Water expansion because of higher temperatures and melting of landlocked ice. Identify climate change as an outcome of the enhanced greenhouse effect. Solve problems related to the enhanced greenhouse effect. Problems could involve volume expansion, specific heat capacity and latent heat. Identify some possible solutions to reduce the enhanced greenhouse effect. -Increase power production efficiency. -Replace coal and oil with natural gas. -Use combined heating and power systems (CHP). -Use renewable energy and nuclear power. -Use CO2 capture and storage. -Use hybrid vehicles. Discuss international efforts to reduce the enhanced greenhouse effect. -Intergovernmental panel on climate change (IPCC), -Kyoto Protocol, -Asia-Pacific Partnership on Clean Development and Climate (APPCDC). O P T I O N S - STANDARD LEVEL (and part of higher level) B Quantum physics and nuclear physics B1 Quantum physics The Quantum Nature of Radiation B.1.1 Describe the photoelectric effect. B.1.2 Describe the concept of the photon and use it to explain the photoelectric effect. B.1.3 B.1.4 Describe and explain an experiment to test the Einstein model. Solve problems involving the photoelectric effect. -Be able to apply E = hf, and hf = + Emax and hf = hfo + eV. The Wave Nature of Matter B.1.5 Describe the de Broglie hypothesis and the concept of matter waves. Be aware of the wave-particle duality of matter and radiation. B.1.6 Outline an experiment to verify the de Broglie hypothesis. An outline of the Davisson-Germer experiment will suffice. B.1.7 Solve problems involving matter waves. -You should be able to calculate the wavelength of an electron after acceleration through a given potential difference. -Be able to apply = h/p. Atomic Spectra and Atomic Energy States B.1.8 Outline a laboratory procedure for producing and observing atomic spectra. -Know what emission and absorption spectra are. B.1.9 Explain how atomic spectra provide evidence for the quantization of energy in atoms. B.1.10 Calculate wavelengths of spectral lines from energy level differences and vice versa. B.1.11 Explain the origin of atomic energy levels in terms of the ‘electron in a box’ model. -Be able to derive EK = n2h2/(8meL2) for an electron in a box of length L. B.1.12 Outline the Schrodinger model of the hydrogen atom. -This model assumes the electrons in the atom may be described by wavefunctions. The electron has an undefined position, but the square of the amplitude of the wavefunction gives the probability of finding an electron at a particular point. B.1.13 Outline the Heisenberg uncertainty principle with regard to positionmomentum and time-energy. Be able to apply xp h/4 and Et h/4. B2 B.2.1 B.2.2 B.2.3 Nuclear physics Explain how the radii of nuclei may be estimated from charges particle scattering experiments. Describe how the masses of nuclei may be determined using a Bainbridge mass spectrometer. Describe one piece of evidence for the existence of nuclear energy levels. -For example alpha particles and gamma rays emitted by decaying nuclei all have discrete (quantized) energies. Radioactive decay B.2.4 Describe beta+ (+) decay including the existence of the neutrino. -You should know that beta particle energy spectra are continuous, and that the neutrino was postulated to account for these spectra. B.2.5 State the radioactive decay law as an exponential function and define the decay constant. -You should know that the decay constant is defined as the probability of decay of a nucleus per unit time. B.2.6 Derive the relationship between decay constant and half-life. -Be able to show that Thalf = ln2/. B.2.7 Outline methods for measuring the half-life of an isotope. B.2.8 Solve problems involving radioactive half-life. -Be able to use Thalf = ln2/ and A = -N/t and N = Noe -t and A = N =Noe -t. D Relativity and particle physics D1 D.1.1 D.1.2 D.1.3 Introduction to relativity Describe what is meant by a frame of reference. Describe what is meant by a Galilean transformation. Solve problems involving relative velocities using the Galilean transformation equations. D2 D.2.1 Concepts and postulates of special relativity Describe what is meant by an inertial frame of reference. -Know that it is a non-accelerating frame of reference. State the two postulates of special relativity. -The laws of physics are the same in all inertial reference frames. -The speed of light is the same in all inertial reference frames. Discuss the concept of simultaneity. D.2.2 D.2.3 D3 Relativistic kinematics Time dilation D.3.1 Describe the concept of a light clock. -This is a beam of light reflected between two mirrors, as in our notes. D.3.2 Define proper time interval. -This is the time as measured by an observer in the rest frame of the two events. D.3.3 Derive the time dilation formula. Be able to derive t = /to, where = sqrt[1/(1-v2/c2)]. D.3.4 D.3.5 Sketch and annotate a graph showing the variation with relative velocity of the Lorentz factor . Solve problems involving time dilation. Be able to use t = /to, where = sqrt[1/(1-v2/c2)]. Length contraction D.3.6 Define proper length. L=Lo/. D.3.7 Describe the phenomenon of length contraction. -The derivation of the length contraction formula is not required. D.3.8 Solve problem involving length contraction. D4 Particles and interactions Description and classification of particles D.4.1 State what is meant by an elementary particle. -Particles are called elementary if they have no internal structure. D.4.2 Identify elementary particles. The classes of elementary particle are quarks, leptons and exchange particles. The Higgs particle could be elementary. D.4.3 Describe particles in terms of mass and various quantum numbers. -Consider electric charge, spin, strangeness, color, lepton number, and baryon number. D.4.4 Classify particles according to spin. -A quark may have spin-up or -down. Spin-up is +1/2, and -down is -1/2. -Baryons have three quarks. The spin of a baryon can be +/-1/2 or +/-3/2. -Particles whose total spin is a half-integer is called a fermion. Baryons and quarks are fermions. Fermions obey Pauli’s exclusion principle. -Mesons have two quarks. Thus the spin of a meson can be 0, or +/-1, depending on whether the quarks’ spins add or cancel. -Particles whose total spin is an integer are called bosons. Bosons do NOT obey Pauli’s exclusion principle. D.4.5 State what is meant by an antiparticle. -An antiparticle has the same mass as its corresponding particle, but all of its quantum numbers are the opposite. -If a particle has a charge of 1, its antiparticle has a charge of -1. D.4.6 State the Pauli exclusion principle. -No two fermions may have all of their quantum numbers the same. Fundamental interactions D.4.7 List the fundamental interactions. -They are strong, electromagnetic, weak (electro-weak) and gravitational. D.4.8 Describe the fundamental interactions in terms of exchange particles. -Electromagnetic: Photon (). -Strong: Gluon (g). -Weak: W+, W-, and Z particles. D.4.9 -Gravity: Graviton (still not observed...). Discus the uncertainty principle for time-energy in the context of particle creation. -Be able to explain how Et h/4 allows for the creation of particles out of the void according to E = mc2. Feynman diagrams D.4.10 Describe what is meant by a Feynman diagram. -Feynman diagrams are a graphic representation of the probability of a particular particle reaction. D.4.11 Discuss how a Feynman diagram may be used to calculate probabilities for fundamental processes. D.4.12 Describe what is meant by a virtual particle. -Be able to explain how Et h/4 allows for the creation of particles out of the void, but for such a short time interval that the particle can not be observed. -The exchange particles responsible for the strong and the weak forces are virtual. D.4.13 Apply the formula for the range R for interactions involving the exchange of a particle. Be able to use R=h/(4mc). D.4.14 Describe pair annihilation and pair production through Feynman diagrams. -For pair production, a photon becomes a particle/anti-particle pair. -For annihilation, a particle/anti-particle pair becomes a photon. D.4.15 Predict particle processes using Feynman diagrams. D5 D.5.1 D.5.2 D.5.3 D.5.4 Quarks List the six types of quark. -They are u-up, d-down, c-charm, s-strange, t-top, b-bottom (and each has an antiquark). State the content, in terms of quarks and antiquarks, of hadrons (that is, baryons and mesons). -Hadrons are particles that are made of quarks. -Baryons consist of three quarks (or three anti-quarks) -Mesons consist of a quark and an antiquark. State the quark content of the proton and the neutron. -A proton is uud, and -A neutron is udd. Define baryon number and apply the conservation of baryon number. -Know that baryon number is conserved in all reactions. -Each quark has a baryon number of 1/3, and each anti-quark has a baryon number of -1/3. -Thus the baryon number of all mesons is zero. -The baryon number of a proton (uud) and a neutron (udd) is 1. D.5.5 D.5.6 D.5.7 D.5.8 D.5.9 D.5.10 Deduce the spin structure of hadrons (that is, baryons and mesons). Only an elementary discussion in terms of ‘spin up’ and ‘spin down’ is required. See D.4.4 above for details. Explain the need for color in forming bound states of quarks. -Know that ‘color’ is needed to satisfy the Pauli exclusion principle. -The fact that hadrons have no color is a consequence of confinement. State the color of quarks and gluons. -Color is R-red, G-green, and B-blue (and their anticolors). Outline the concept of strangeness. -Know that the strangeness of a hadron is the number of anti-strange quarks minus the number of strange quarks. -Strangeness is conserved in strong and electromagnetic interactions, but not always in weak interactions. Discuss quark confinement. -Know that isolated quarks and gluons (that is, particles with color) cannot be observed. -Strong (color) interaction increases with separation. -More hadrons are produced when sufficient energy is supplied to isolate a quark. Discuss the interaction that binds nucleons in terms of the color force between quarks. -Know that the interaction between nucleons is the residual interaction between the quarks in the nucleons and that -it is a short-range interaction. A D D I T I O N A L H I G H E R L E V E L T O P I C S (AHL) 9 Motion in fields 9.1 9.1.1 Projectile motion State the independence of the vertical and the horizontal components of velocity for a projectile in a uniform field. -In the absence of air, vx = ux = constant and vy = uy - gt. -Each equation is independent of the other. Describe and sketch the trajectory of projectile motion as parabolic in the absence of air resistance. -Proof of the parabolic nature of the trajectory is not required. Describe qualitatively the effect of air resistance on the trajectory of a projectile. Solve problems on projectile motion. Problems may involve projectiles launched horizontally or at any angle above or below the horizontal. Applying conservation of energy may provide a simpler solution to some problems than using projectile motion kinematic equations. 9.1.2 9.1.3 9.1.4 THESE EQUATIONS ARE NOT IN THE PHYSICS DATA BOOKLET. x = xo + vxot, vx = vxo = vo cos = constant y = yo + vyot – (1/2)gt2, vyo = vosin vy = vyo - gt vy2 = vyo2 – 2gy. 9.2 9.2.1 9.2.2 9.2.3 9.2.4 9.2.5 9.2.6 9.2.7 9.2.8 9.2.9 9.3 9.3.1 Gravitational field, potential and energy Define gravitational potential and gravitational potential energy. You should understand the scalar nature of gravitational potential and that the potential at infinity is taken to be zero. -You should understand that the work done in moving a mass between two points in a gravitational field is independent of the path taken. -Gravitational potential energy is given by EP = -Gm1m2/r and -gravitational potential is potential energy per unit mass, or V = EP/m. State and apply the expression for gravitational potential due to a point mass. V = -Gm/r. State and apply the formula relating gravitational field strength to gravitational potential gradient. g = -V/r. Determine the potential due to one or more point masses. Describe and sketch the pattern of equipotential surfaces due to one and two point masses. State the relation between equipotential surfaces and gravitational field lines. -Equipotential surfaces are perpendicular to gravitational field lines. Explain the concept of escape speed from a planet. Derive an expression for the escape speed of an object from the surface of a planet. -Make sure you know the simplifying assumptions in this derivation. Solve problems involving gravitational potential energy and gravitational potential. -Don forget that you need to know all of the formulas from Topic 6, in addition to the Topic 9 ones. Topic 9 is just an extension of Topic 6. EP = mV. Electric field, potential and energy Define electric potential and electric potential energy. -Electric potential energy is given by EP = kq1q2/r and -electric potential is potential energy per unit charge, or 9.3.2 9.3.3 9.3.4 9.3.5 9.3.6 9.3.4 9.4 9.4.1 V = EP/q. -Note the symmetry between the gravitational and electric fields. State and apply the expression for electric potential due to a point charge. V = kq/r. State and apply the formula relating electric field strength to electric potential gradient. E = -V/r. Determine the potential due to one or more point charges. Describe and sketch the pattern of equipotential surfaces due to one and two point charges. State the relation between equipotential surfaces and electric field lines. -Equipotential surfaces are perpendicular to electric field lines. Solve problems involving electric potential energy and electric potential. -Don’t forget that you need to know all of the formulas from Topic 6, in addition to the Topic 9 ones. Topic 9 is just an extension of Topic 6. EP = mV. 9.4.6 Orbital motion State that gravitation provides the centripetal force for circular orbital motion. Derive Kepler’s third law. The third law states that the period squared of a planet in orbit is proportional to the cube of the radius of its orbit. -Here is the derivation: F = GMm/r2 and F = mac and ac = 42r/T2 are all in the Physics Data Booklet. Begin by writing F = m42r/T2 (from F = mac and ac = 42r/T2) and then GMm/r2 = m42r/T2 which will rearrange to T2 = (42/GM) r3, where M is the mass of the Sun. Derive expressions for the kinetic energy, potential energy and total energy of an orbiting satellite. -Don’t forget that Etotal = EP + EK (which in our notation is E = U + K). EK = (1/2)mv2 and EP = -GMm/r. Sketch graphs showing the variation with orbital radius of the kinetic energy, gravitational potential energy and total energy of a satellite. Discuss the concept of weightlessness in orbital motion, in freefall and in deep space. -In freefall you only appear weightless because you are falling as fast as everything around. This includes a cut elevator cable or the space shuttle in orbit (freefall) around the Earth. -Weightlessness in deep space (far from any gravitational bodies) is true weightlessness, because local g is truly zero (W = mg). Your mass is never zero. Solve problems involving orbital motion. 10 Thermal physics 9.4.2 9.4.3 9.4.4 9.4.5 10.1 Thermodynamics Gas Laws 10.1.1 State the equation of state for an ideal gas. You should be aware that an ideal gas is one that has the equation of state PV = nRT and that this equation also defines the universal gas constant R. 10.1.2 Describe the difference between an ideal gas and a real gas. You should be aware of the circumstances in which real gas behavior approximates to ideal gas behavior. You should also appreciate that ideal gases cannot be liquefied. 10.1.3 Describe the concept of the absolute zero of temperature and the Kelvin scale of temperature. Be able to explain and use T(K) = t(°C) + 273. 10.1.4 Solve problems using the equation of state of an ideal gas. PV=nRT is the equation of state of an ideal gas. 10.2 Processes The first law of thermodynamics 10.2.1 Deduce an expression for the work involved in a volume change of a gas at constant pressure. -Know how to show that W = PV. 10.2.2 State the first law of thermodynamics. -Be familiar with the terms system and surroundings. -You should also appreciate that if a system and its surroundings are at different temperatures and the system undergoes a process, the energy transferred by non-mechanical means to or from the system is referred to as thermal energy (heat). -The first law in formula form is Q = U + W, where U is the change in internal energy of the system. 10.2.3 Identify the first law of thermodynamics as a statement of the principle of energy conservation. 10.2.4 Describe the isochoric (isovolumetric), isobaric, isothermal, and adiabatic changes of state of an ideal gas. -Isochoric-constant volume (V=0). -Isobaric-constant pressure (P=0). Isothermal-constant temperature(T=0). Adiabatic-constant heat (Q=0). 10.2.5 Draw and annotate thermodynamic processes and cycles on P-V diagrams. 10.2.6 Calculate from a P-V diagram the work done in a thermodynamic cycle.The work done in a thermodynamic cycle is the area under the P-V graph. Find the work for each part of the cycle and be sure to give it the correct sign (it can be positive or negative). Then sum up the individual works for the total. 10.2.7 Solve problems involving state changes of a gas. 10.3 10.3.1 10.3.2 10.3.3 10.3.4 Second law of thermodynamics and entropy State that the second law of thermodynamics implies that thermal energy cannot spontaneously transfer from a region of low temperature to a region of high temperature. State that entropy is a system property that expresses the degree of disorder of a system. State the second law of thermodynamics in terms of entropy changes. -Knowing that the overall entropy of the universe is increasing is sufficient, and that all natural processes increase the entropy of the universe. Discuss examples of natural processes in terms of entropy changes. You should understand that, although local entropy may decrease, any process will increase the total entropy of the system and surroundings, that is, the universe. 11 Wave phenomena 11.1 11.1.1 Standing (stationary) waves Describe the nature of standing (stationary) waves. Be sure to consider energy transfer, amplitude and phase. Explain the formation of one-dimensional standing waves. Know what nodes and antinodes are, and how they form. Discuss the modes of vibration of strings and air in open and in closed pipes. The lowest-frequency mode is known either as the fundamental or as the first harmonic. The term overtone will not be used. Compare standing waves and traveling waves. Solve problems involving standing waves. Doppler effect Describe what is meant by the Doppler effect. Explain the Doppler effect by reference to wavefront diagrams for moving-detector and moving-source situations. -Know that spherical wavefronts bunch up in the direction of relative motion, and spread out in the opposite direction. Apply the Doppler effect equations for sound. -For moving source f’ = fv/(v +/- uo) . -For moving observer f’ = f(v +/- uo)/v. Solve problems on the Doppler effect for sound. -Problems will not include situations where both the source and the detector are moving. Solve problems on the Doppler effect for electromagnetic waves using the approximation f = vf/c. -Know that this approximation may be used only when v<<c. Outline an example in which the Doppler effect is used to measure speed. -Know how a radar gun works, or Doppler radar. 11.1.2 11.1.3 11.1.4 11.1.5 11.2 11.2.1 11.2.2 11.2.3 11.2.4 11.2.5 11.2.6 11.3 Diffraction Diffraction at a single slit 11.3.1 Sketch the variation with angle of diffraction of the relative intensity of light diffracted at a single slit. 11.3.2 diffraction pattern produced at a single slit. 11.3.3 Solve problems involving single-slit diffraction. Be able to apply the formula = /b. 11.4 11.4.1 11.4.2 11.4.3 11.4.4 11.5 11.5.1 11.5.2 11.5.3 11.5.4 11.5.5 Resolution Sketch the variation with angle of diffraction of the relative intensity of light emitted by two point sources that has been diffracted at a single slit. Sketch the variation where the diffraction patterns are well-resolved, just resolved and not resolved. State the Rayleigh criterion for images of two sources to be just resolved. Know that the criterion for a circular aperture is = 1.22/b. Describe the significance of resolution in the development of devices such as CDs and DVDs, the electron microscope and radio telescopes. -Resolution depends on the wavelength of the laser that is used to read the pits in a CD or a DVD. The shorter the wavelength, the smaller the pits can be. This translates to more data storage. -The same principle applies to electron microscopes. The smaller the wavelength the smaller the object to be observed can be. Solve problems involving resolution. Problems could involve the human eye and optical instruments. Polarization Describe what is meant by polarized light. -Unpolarized light has infinitely many planes of polarization. -Polarized light has a single plane of polarization. Describe polarization by reflection. -If reflected and refracted light rays make an angle of 90° (see Snell’s law) then the reflected light ray will be polarized. State and apply Brewster’s law. n = tan , where n is the index of refraction of the glass (or other refracting material) and is the angle of incidence at which complete polarization occurs. Explain the terms polarizer and analyzer. -A polarizer initially polarizes unpolarized light. -An analyzer is a second polarizing material that can be rotated to determine the plane of polarization of an already-polarized ray of light. Calculate the intensity of a transmitted beam of polarized light using Malus law. I = Io cos2 , 11.5.8 11.5.9 11.5.10 where Io is the intensity of incident polarized light, is the angle between the incident plane of polarization and the analyzer plane of polarization, and I is the intensity of the transmitted ray. Describe what is meant by an optically active substance. -Know that these substances rotate the plane of polarization of incident polarized light. Describe the use of polarization in the determination of the concentration of certain solutions. Outline qualitatively how polarization may be used in stress analysis. Outline qualitatively the action of liquid-crystal displays (LCDs). Solve problems involving the polarization of light. 12 Electromagnetic induction 12.1 12.1.1 Induced electromotive force (emf) Describe the inducing of an emf by relative motion between a conductor and a magnetic field. Since conductors contain many free-to-move electrons, and since the motion of a charge through a magnetic field places a force on that charge according to F = qvBsin, motion of a conductor through a magnetic field will produce an emf. Derive the formula for the emf induced in a straight conductor moving in a magnetic field.You should be able to derive the equation emf = BLv without using Faradays law. L is the length of conductor moving through the B-field. Define magnetic flux and magnetic flux linkage. -Magnetic flux is given by = BAcos where B is the magnetic field, A is the area through which the field travel, and is the angle between the direction of the B-field and the normal vector to the area. Describe the production of an induced emf by a time-changing magnetic flux. A coil of a generator rotating in a magnetic field is an example. State Faraday’s law and Lenz’s law. -Faraday’s law-the induced emf in a coil of wire is proportional to the number of loops and the time rate of change of magnetic flux = -/t. -The reason there is a negative of Faraday’s law is because of Lenz’s law, which states that an induced emf gives rise to a current whose magnetic field opposes the change in magnetic flux that produced it. Solve electromagnetic induction problems. 11.5.6 11.5.7 12.1.2 12.1.3 12.1.4 12.1.5 12.1.6 12.2 12.2.1 Alternating current Describe the emf induced in a coil rotating within a uniform magnetic field. -Know that the induced emf is sinusoidal if the rotation is at a constant speed. 12.2.2 12.2.3 12.2.4 12.2.5 12.2.6 12.2.7 12.2.8 12.2.9 12.3 12.3.1 12.3.2 12.3.3 Explain the operation of a basic alternating current (ac) generator. Coils of wire are rotated by mechanical means in a magnetic field producing a sinusoidal emf. Describe the effect on the induced emf of changing the generator frequency. You will be expected to compare the output from generators operating at different frequencies by sketching appropriate graphs. Note that the defining formula = 2BAf sin (2ft) shows that is proportional to f. Discuss what is meant by the root mean squared (rms) value of an alternating current or voltage. -You should know that the rms value of an alternating current (or voltage) is that value of the direct current (or voltage) that dissipates power in a resistor at the same rate. -The rms value is also known as the rating. State the relation between peak and rms values for sinusoidal currents and voltages. Irms = Io/2. Vrms = Vo/2, where Io and Vo are the peak values. Solve problems using peak and rms values. Solve ac circuit problems for ohmic resistors. R = Vo/Io = Vrms/Irms. Describe the operation of ideal transformers. Is/Ip = Vp/Vs = Np/Ns. The p and s subscripts are primary and secondary. Solve problems on the operation of ideal transformers. Transmission of electrical power Outline the reasons for power losses in transmission lines and real transformers. -Thermal power loss, also known as I2R loss, or heat loss, is due to the resistance of the wire carrying the current in the transmission lines, or stepping of the voltages in transformers, or flux linkage losses in transformers. Explain the use of high-voltage step-up and step-down transformers in the transmission of electrical power. -Be aware that for economic reasons there is no ideal value of voltage for electrical transmission. Solve problems on the operation of real transformers and power transmission. -For transformers: Is/Ip = Vp/Vs = Np/Ns. The p and s subscripts are primary and secondary. -For transmission lines: Pmax = IoVo and Pav = (1/2)IoVo. 12.3.4 12.3.5 13 Suggest how extra-low-frequency electromagnetic fields, such as those created by electrical appliances and power lines, induce currents in the human body. The frequency is resonant with cells in the body, which contain ions (charged molecules) which then respond to the magnetic flux change of power lines, etc. Discuss some of the possible risks involved in living and working near high-voltage power lines. -Be aware that current experimental evidence suggests that low frequency fields do not harm genetic material. -But understand that the risks attached to the inducing of current in the body are not fully understood. -These risks are likely to be dependent on current (density), frequency and length of exposure. Quantum physics and nuclear physics 13.1 Quantum physics The quantum nature of radiation 13.1.1 Describe the photoelectric effect. 13.1.2 Describe the concept of the photon, and use it to explain the photoelectric effect. Be able to explain why the wave model of light is unable to account for the photoelectric effect, and be able to describe and explain the Einstein model. 13.1.3 Describe and explain an experiment to test the Einstein model. Millikans experiment involving the application of a stopping potential would be suitable. 13.1.4 Solve problems involving the photoelectric effect. Be able to apply E = hf, hf = hfo+eV and hf = o + Emax. The wave nature of matter 13.1.5 Describe the de Broglie hypothesis and the concept of matter waves. -Be aware of wave/particle duality (the dual nature of both matter and radiation). 13.1.6 Outline an experiment to verify the de Broglie hypothesis. -A brief outline of the Davisson-Germer experiment will suffice. -This experiment projected electrons through a crystal lattice structure and the electrons diffracted in a wavelike manner. 13.1.7 Solve problems involving matter waves. -For example, you should be able to calculate the wavelength of electrons after acceleration through a given potential difference. -Be able to use p = h/. Atomic spectra and atomic energy states 13.1.8 Outline a laboratory procedure for producing and observing atomic spectra. 13.1.9 13.1.10 13.1.11 13.1.12 13.1.13 13.2 13.2.1 13.2.2 13.2.3 -For example, applying a voltage to a gas-discharge tube and observing the emitted light spectra using a spectroscope. -Fluorescent light bulbs are examples of gas-discharge tubes. Explain how atomic spectra provide evidence for the quantization of energy in atoms. -For example the emission spectrum of a hydrogen gas-discharge tube shows the very specific and separated allowed energies, revealing the quantum nature of atoms. Calculate the wavelengths of spectral lines from energy level differences, and vice versa. -Be able to use E = hf and c = f to show that E=hc/. Explain the origin of atomic energy levels in terms of the electron in a box model. The model assumes that if an electron is confined to move in one dimension by a box, the de Broglie waves associated with the electron will be standing waves of wavelength 2L/n, where L is the length of the box and n is any positive integer. You should be able to show that the kinetic energy EK of the electron in the box is EK = n2h2/(8meL2). Outline the Schrodinger model of the hydrogen atom. -The model assumes that electrons in the atom may be described as wavefunctions. The electron has an undefined position, but the square of the amplitude of the wavefunction gives the probability of finding the electron at a particular point. Outline the Heisenberg uncertainty principle with regard to positionmomentum and time-energy. -You should know that the conjugate quantities position-momentum and time-energy cannot be known precisely at the same instant. -You should know of the link between the uncertainty principle and the de Broglie hypothesis. For example, you should know that if a particle has a uniquely defined de Broglie wavelength, then its momentum is known precisely but all knowledge of its position is lost. -Be able to apply xp h/(4) and Et h/(4). Nuclear physics Explain how the radii of nuclei may be estimated from charges particle scattering experiments. -Use of energy conservation for determining closest-approach distances for Coulomb scattering experiments is sufficient. Think Rutherford. Describe how the masses of nuclei may be determined using a Bainbridge mass spectrometer. -Know how to draw a schematic diagram of the Bainbridge mass spectrometer. -Know that nuclear mass differences provide evidence for the existence of isotopes. Describe one piece of evidence for the existence of nuclear energy level -Know that alpha () particles produced by the decay of a nucleus have discrete energies; gamma-ray (-ray) spectra are discrete. -Know that the nucleus, like the atom, is a quantum system, and as such, has discrete energy levels. Radioactive decay 13.2.4 Describe + decay, including the existence of the neutrino. -Know that the energy spectra are continuous, and that the neutrino was postulated to account for these spectra. 13.2.5 State that the radioactive decay law is an exponential function and define the decay constant. -Know that the decay constant is defined as the probability of decay of a nucleus per unit time. 13.2.6 Derive the relationship between the decay constant and the half-life. 13.2.7 Outline methods for measuring the half-life of an isotope. -Know the principles of measurement for both long and short half-lifes. 13.2.8 Solve problems involving radioactive half-life. 14 Digital technology 14.1 14.1.1 14.1.2 14.1.3 14.1.4 14.1.5 14.1.6 14.1.7 Analogue and digital signals Solve problems involving the conversion between binary numbers and decimal numbers. -Know that the term bit is binary digit and it can be a zero or a one. -Know that the least significant bit (LSB) is the rightmost one, and that the most significant bit (MSB) is the leftmost one. -Problems will be limited to a maximum if five bits in digital numbers. Describe different means of storage of information in both analog and digital forms. -Know about LPs, cassette tapes, floppy disks, hard disks, CDs, DVDs, and so on. Explain how interference of light is used to recover information stored on a CD. -Know that destructive interference occurs when light is reflected from the edge of a pit. Calculate the appropriate depth of a pit given the wavelength of the laser light. Solve problems on CDs and DVDs related to storage capacity. Discuss the advantage of storage of information in digital rather than analog form. -Consider quality, reproducibility, retrieval speed, portability of stored data and manipulation of data. Discuss the implications for society of ever-increasing capability of data storage. -Consider moral, ethical, social, economic, and environmental implications. 14.2 14.2.1 14.2.2 14.2.3 14.2.4 14.2.5 14.2.6 14.2.7 14.2.8 14.2.9 14.2.10 14.2.11 Data capture; digital imaging using charge-coupled divices (CCDs) Define capacitance. -Capacitance is the ability of an electronic component to store electric charge. Describe the structure of a charge-coupled device (CCD). -Students should know that a CCD is a silicon chip divided into small areas called pixels (picture elements). Each pixel can be considered to behave like a capacitor. Explain how incident light causes charge to build up within a pixel. -Think and use photoelectric effect. Outline how the image on a CCD is digitized. -You are required to know that an electrode measures the potential difference developed across each pixel and this is then converted to a digital signal. The pixel position is also digitally stored. Define quantum efficiency of a pixel. -Quantum efficiency is the ratio of the number of photoelectrons emitted to the number of photon incident on the pixel. Define magnification. -Know that magnification is the ratio of the length of the image on the CCD to the length of the object. State that two points on an object may be just resolved on a CCD if the images of the points are at least two pixels apart. Discuss the effects of quantum efficiency, magnification and resolution on the quality of the processed image. Describe a range of practical uses of a CCD, and list some advantages compared with the use of film. -Know that CCDs are used for image capturing in a large range of the electromagnetic spectrum. -Consider items such as digital cameras, video cameras, telescopes (including the Hubble Space Telescope), and medical X-ray imaging. Outline how the image stored on a CCD is retrieved. Solve problems involving the use of CCDs. O P T I O N S - HIGHER LEVEL H Relativity H1 Introduction to relativity Frames of reference H.1.1 Describe what is meant by a frame of reference. H.1.2 Describe what is meant by a Galilean transformation. H.1.3 Solve problems involving relative velocities using the Galilean transformation equations. H2 H.2.1 Concepts and postulates of special relativity Describe what is meant by an inertial reference frame. -Know that it is a non-accelerating frame of reference. State the two postulates of special relativity. -The laws of physics are the same in all inertial reference frames. -The speed of light is the same in all inertial reference frames. Discuss the concept of simultaneity. H.2.2 H.2.3 H3 Relativistic kinematics Time dilation H.3.1 Describe the concept of a light clock. This is a beam of light reflected between two mirrors, as in our notes. H.3.2 Define proper time interval. H.3.3 Derive the time dilation formula. -Be able to derive t = to, where = SQRT[ 1/(1-v2/c2) ]. H.3.4 Sketch and annotate a graph showing the variation with relative velocity of the Lorentz factor. H.3.5 Solve problems involving time dilation. -Be able to use t = to, where = SQRT[ 1/(1-v2/c2) ]. Length contraction H.3.6 Define proper length. H.3.7 Describe the phenomenon of length contraction. -The derivation of the length contraction formula is not required. H.3.8 Solve problem involving length contraction. L=Lo/. H4 Some consequences of special relativity The twin paradox H.4.1 Describe how the concept of time dilation leads to the twin paradox. -Different observers’ versions of the time taken for a journey at speed close to the speed of light might be compared. H.4.2 Discuss the Hafele-Keating experiment. Velocity addition H.4.3 Solve one-dimensional problems involving the relativistic addition of velocities. -The derivation of the formula is not required. -Be able to apply ux’ = (ux - v)/(1 - uxv/c2). Mass and energy H.4.4 State the formula representing the equivalence of mass and energy. -Be able to use E = mc2. H.4.5 Define rest mass. -The rest mass of an object is the mass as measured in a frame of reference in which the mass is NOT moving. -Rest mass is an invariant quantity. -Be familiar with the units MeVc-2 for mass, as well as kg. H.4.6 Distinguish between the energy of a body at rest and its total energy when it is moving. -At rest E = Eo = moc2. -At speed E = moc2. H.4.7 Explain why no object can ever attain the speed of light in a vacuum -The mass of the object increases to infinity if it reaches c, and this cannot happen because there is not enough energy to move an infinite mass. H.4.8 Determine the total energy of an accelerated particle. -Be able to calculate, for example, the total energy of an electron after it has been accelerated through a known potential difference. H5 H.5.1 H.5.2 H.5.3 H.5.4 H.5.5 H6 H.6.1 H.6.2 H.6.3 Evidence to support special relativity Discuss muon decay as experimental evidence to support special relativity. -A muon is an unstable particle that is a product of cosmic rays that strike the Earth reacting with the atmosphere. Its lifetime at rest is shorter than the time it takes to reach us from the outer atmosphere, so the implication is that because it is moving extremely fast, time dilation allows it to survive on its trip to the ground. Solve some problems involving the muon decay experiment. Outline the Michelson-Morley experiment and its implication. -Be able to outline the principles of the Michelson interferometer using a simple sketch of the apparatus. Discuss the result of the Michelson-Morley experiment and its implication. -The implication is that the ether does not exist and the result is consistent with the constancy of the speed of light is the accepted explanation. Outline an experiment that indicates that the speed of light in vacuum is independent of its source. -You should familiar with pion decay experiments involving the decay of a fast-moving pion into two gamma-ray ( -ray) photons Relativistic momentum and energy Apply the relation for the relativistic momentum p = mou of particles. -Be familiar with momentum being expressed using the unit MeV c-1. Apply the formula EK = ( - 1)moc2 for the relativistic kinetic energy of a particle. Be able to use E2 = p2c2 + moc2 also. Solve problems involving relativistic momentum and energy. -Know how to calculate, for example, the kinetic energy, total energy, speed and momentum of an accelerated particle and for particles produced in reactions. H7 General relativity The equivalence principle H.7.1 Explain the difference between the terms gravitational mass and inertial mass. -Gravitational mass is the mass used to calculate the gravitational attraction to another mass and satisfies F = GMm/r2. -Inertial mass is that mass that resists a change in speed and satisfies F = ma. -It turns out that the gravitational mass is equivalent to the inertial mass. This is why all masses fall at the same acceleration. H.7.2 Describe and discuss Einstein’s principle of equivalence. -Know about Einstein’s closed elevator thought experiment. -The principle of equivalence states that an accelerated reference frame is equivalent to a reference frame in a gravitational field. H.7.3 Deduce that the principle of equivalence predicts the bending of light rays in a gravitational field. H.7.4 Deduce that the principle of equivalence predicts that time slows down near a massive body. Spacetime H.7.5 Describe the concept of spacetime. H.7.6 State that moving objects follow the shortest path between two points in spacetime. H.7.7 Explain gravitational attraction in terms of warping of spacetime by matter. Black holes H.7.8 Describe black holes. -Know that black holes are a region of spacetime with extreme curvatures due to the presence of a mass. H.7.9 Define the term Schwarzschild radius. -Schwarzschild radius is the radius a body would have to be reduced to in order to make it a black hole. -Essentially it is the radius needed to make the escape velocity c. H.7.10 Calculate the Schwarzschild radius. -Be able to apply Rs = 2GM/c2. H.7.11 Solve problems involving time dilation close to a black hole. Gravitational red shift H.7.12 Describe the concept of gravitational red shift. -Know that the wavelength of light passing in the vicinity of a massive object is stretched. H.7.13 H.7.14 H8 H.8.1 H.8.2 H.8.3 J -This means that the wavlength gets shifted toward the red side of the visible spectrum (the long wavelengths). -Know that gravitational red shift is a prediction of the general theory of relativity. Solve problems involving frequency shifts between different points in a uniform gravitational field. -Be able to apply f/f = gh/c2. Solve problems using the gravitational time-dilation formula. -Be able to apply t = to/(1 - Rs/r)1/2. Evidence to support general relativity Outline an experiment for the bending of electromagnetic waves by a massive object. -An outline of the principles used in, for example, Eddingtons measurements during the 1919 eclipse of the sun is sufficient. -Know also the question of ethics and the limitations of data that this historical experiment illustrates. Describe gravitational lensing. Outline an experiment that provides evidence for gravitational red-shift. The Pound-Rebka experiment will suffice. Particle physics J1 Particles and interactions Description and classification of particles J.1.1 State what is meant by an elementary particle. -Particles are called elementary if they have no internal structure. J.1.2 Identify elementary particles. -The classes of elementary particle are quarks, leptons and exchange particles. -The Higgs particle could be elementary. J.1.3 Describe particles in terms of mass and various quantum numbers. -Consider electric charge, spin, strangeness, color, lepton number, and baryon number. J.1.4 Classify particles according to spin. -A quark may have spin-up or spin-down. Spin-up is +1/2, and spin-down is -1/2. -Baryons have three quarks. The spin number of a baryon can be +/-1/2 or +/-3/2. -Particles whose total spin is a half-integer is called a fermion. -Baryons and quarks are fermions. -Fermions obey Pauli’s exclusion principle. -Mesons have two quarks. Thus the spin number of a meson can be 0, or +/-1, depending on whether the quarks’ spins add or cancel. J.1.5 J.1.6 -Particles whose total spin is an integer are called bosons. -Bosons do NOT obey Pauli’s exclusion principle. State what is meant by an antiparticle. -An antiparticle has the same mass as its corresponding particle, but all of its quantum numbers are the opposite. -If a particle has a charge of 1, its antiparticle has a charge of -1. State the Pauli exclusion principle. -No two fermions may have all of their quantum numbers the same. Fundamental interactions J.1.7 List the fundamental interactions. -They are strong, electromagnetic, weak (electro-weak) and gravitational. J.1.8 Describe the fundamental interactions in terms of exchange particles. -Electromagnetic -photon (). -Strong-Gluon (g). -Weak-W+, W-, and Z0 particles. -Gravity- graviton (still not observed...). J.1.9 Discus the uncertainty principle for time-energy in the context of particle creation. -Be able to explain how Et h/(4) allows for the creation of particles out of the void according to E = mc2. Feynman diagrams J.1.10 Describe what is meant by a Feynman diagram. -Feynman diagrams are a graphic representation of the probability of a particular particle reaction. J.1.11 Discuss how a Feynman diagram may be used to calculate probabilities for fundamental processes. J.1.12 Describe what is meant by a virtual particle. -Be able to explain how Et h/(4) allows for the creation of particles out of the void, but for such a short time interval that the particle can not be observed. -The exchange particles responsible for the strong and weak forces are virtual. J.1.13 Apply the formula for the range R for interactions involving the exchange of a particle. -Be able to use R = h/(4mc). -Applications include Yukawas prediction of the pion or determination of the masses of the W+,W-, and the Z0 from knowledge of the range of the weak interaction. J.1.14 Describe pair annihilation and pair production through Feynman diagrams. -For pair production, a photon becomes a particle/anti-particle pair. -For annihilation, a particle/anti-particle pair becomes a photon. J.1.15 Predict particle processes using Feynman diagrams. J2 Particle accelerators and detectors Particle accelerators J.2.1 Explain the need for high energies in order to produce particles of large mass -Because of E = mc2, the bigger the mass, the more energy you need. J.2.2 Explain the need for high energies in order to resolve particles of small size. -Know that to resolve a particle of size d, the de Broglie wavelength = h/p of the particle used to scatter from it must be of the same order of magnitude as d. -Identify this with the connection to diffraction, which occurs only if the aperture has a comparable width to the wavelength of the incoming wave. J.2.3 Outline the structure and operation of a linear accelerator and of a cyclotron. -The linear accelerator accelerate the particles in stages/bursts in a straight line, each burst accelerating it to a higher velocity. -The cyclotron does the same thing, but allows the particle to keep on using the same stages over and over, instead of only once (as in the linear accelerator). J.2.4 Outline the structure and explain the operation of the synchrotron. -Be able to explain how the charged beams are accelerated, why the magnetic field must vary and why the ring has a large radius. J.2.5 State what is meant by bremsstrahlung (braking) radiation. J.2.6 Compare the advantages and disadvantages of linear accelerators, cyclotrons and synchrotrons. J.2.7 Solve problems related to the production of particles in accelerators. -These include the total energy of the particle in terms of its mass and kinetic energy, and the total energy available from the collision of a particle with a stationary object. -Be able to apply E = hf, E = mc2 + EK and Ea2 = 2Mc2E + (Mc2)2 + (mc2)2. Particle detectors J.2.8 Outline the structure and operation of a bubble chamber, the photomultiplier and the wire chamber. J.2.9 Outline international aspects of research into high-energy particle physics. -Be aware that governments need to collaborate to construct and operate large-scale research facilities. -There are very few accelerator facilities, for example, CERN, DESY, SLAC, Fermilab and Brookhaven. -Results are disseminated and shared by scientists in many countries. J.2.10 Discuss the economic and ethical implications of high-energy particle physics research. -Be aware that even at the height of the Cold War, Western and Soviet scientists collaborated in the field of particle physics. J3 J.3.1 J.3.2 J.3.3 J.3.4 J.3.5 J.3.6 J.3.7 J.3.8 J.3.9 J.3.10 J4 J.4.1 Quarks List the six types of quark. -They are u-up, d-down, c-charm, s-strange, t-top, and b-bottom . -Each has an antiquark. State the content, in terms of quarks and antiquarks, of hadrons (that is, baryons and mesons). -Hadrons are particles that are made of quarks. -Baryons consist of three quarks (or three anti-quarks). -Mesons consist of a quark and an antiquark. State the quark content of the proton and the neutron. A proton is uud. -A neutron is udd. Define baryon number and apply the conservation of baryon number. -Know that baryon number is conserved in all reactions. -Each quark has a baryon number of 1/3, and each anti-quark has a baryon number of -1/3. -Thus the baryon number of all mesons is zero. -The baryon number of a proton (uud) and a neutron (udd) is 1. Deduce the spin structure of hadrons (that is, baryons and mesons). -Only an elementary discussion in terms of ‘spin up’ and ‘spin down’ is required. -See D.4.4 above for details. Explain the need for color in forming bound states of quarks. -Know that ‘color’ is needed to satisfy the Pauli exclusion principle. -The fact that hadrons have no color is a consequence of confinement. State the color of quarks and gluons. -Color is R-red, G-green, and B-blue (and their anticolors). Outline the concept of strangeness. -Know that the strangeness of a hadron is the number of anti-strange quarks minus the number of strange quarks. -Strangeness is conserved in strong and electromagnetic interactions, but not always in weak interactions. Discuss quark confinement. -Know that isolated quarks and gluons (that is, particles with color) cannot be observed. Strong (color) interaction increases with separation. More hadrons are produced when sufficient energy is supplied to isolate a quark. Discuss the interaction that binds nucleons in terms of the color force between quarks. -Know that the interaction between nucleons is the residual interaction between the quarks in the nucleons and that it is a short-range interaction. Leptons and the standard model State the three-family structure of quarks and leptons in the standard model -Family 1: quarks u, d; leptons e, e (up quark, down quark, electron, electron neutrino) J.4.2 J.4.3 J.4.4 J5 J.5.1 J.5.2 J.5.3 J.5.4 J.5.5 -Family 2: quarks c, s; leptons , (charm quark, strange quark, muon, muon neutrino) -Family 3: quarks t, b; leptons , (top quark, bottom quark, tau particle, tau neutrino) -All of these particles have corresponding anti-particles. State the lepton number of the leptons in each family. -Each of the six leptons ( e, e, , , , ) is given a lepton number of L = 1 (or L = -1 if it is an antiparticle). -Each of the leptons has a baryon number of B = 0. Solve problems involving conservation laws in particle reactions. -You should know that electric charge, total energy, momentum, baryon number and family lepton number are conserved in all particle reactions. --Strangeness is conserved in strong and electromagnetic interactions, but not always in weak interactions. Evaluate the significance of the Higgs particle (or Higgs boson). -Know that particles acquire mass as a result of interactions involving the Higgs boson. -To date the Higgs particle has not been detected. -CERN has been designed to have high enough energies to determine if the Higgs boson exists. -If it does not exist, the standard model will not extend to the realm of general relativity. Experimental evidence for the quark and the standard model State what is meant by deep inelastic scattering. -Low energy electrons striking protons did not penetrate the internal structure of the proton. They scattered at shallow angles. -However high energy electrons (20 GeV) were shown to scatter in such a way that the internal structure of the proton was revealed to contain three very small charged particles (later called quarks). -This process was named deep inelastic scattering and was studied by Feynman and Bjorken in 1968. Analyze the results of deep inelastic scattering. -Know that these experiments provided evidence for the existence of quarks, gluons and color. Describe what is meant by asymptotic freedom. -It is sufficient for you to know that the strength of the strong interaction decreases as the energy available for the interaction increases. Describe what is meant by neutral current. -A simple description in terms of processes involving the Z0 exchange is sufficient. Describe how evidence of a neutral current is evidence for the standard model. -Know that only the standard model predicts weak interaction processes involving the exchange of a massive, neutral particle called the Z0 boson. J6 J.6.1 J.6.2 J.6.3 J.6.4 J.6.5 Cosmology and strings State the order of magnitude of the temperature change of the universe since the Big Bang. -The temperature of the universe was 1032 K at 10-43 seconds after the Big Bang and is 2.7 K at present. -Penzias and Wilson discovered this cosmic background radiation. Solve problems involving particle interactions in the early universe. -Know that in the early universe temperatures were so high that particleantiparticle pairs were created all the time. -The time range is 10-43 s to 10-4 s. -Be able to predict the temperature at which electron-positron pairs can be produced using EK = (3/2)kT. -Know the temperature at which nucleosynthesis can occur (1010 K to 3000K). -The time range is 10-4 s to 1013 s (106 years) after the Big Bang. -Know when the universe became transparent to radiation (3000K to 2.7K). -The time range is 1013 s to present. State that the early universe contained almost equal numbers of particles and antiparticles. -This is because pair production is symmetric. Suggest a mechanism by which the predominance of matter over antimatter has occurred. -A simple explanation in terms of the impossibility of photons materializing into particle-antiparticle pairs once the temperature fell below a certain value is all that is required. Describe qualitatively the theory of strings. -You should be aware that the failure to reconcile gravitation with quantum theory has created the idea of the string as the fundamental building block of the matter. -The known fundamental particles are modes of vibrations (resonances) of the string similar to the harmonics of an ordinary string. -Most string theories require the introduction of an additional 6-7 spatial dimensions to the current 3 spatial and 1 time dimensions of spacetime.