1 - Mr. Markic's Chemistry
advertisement
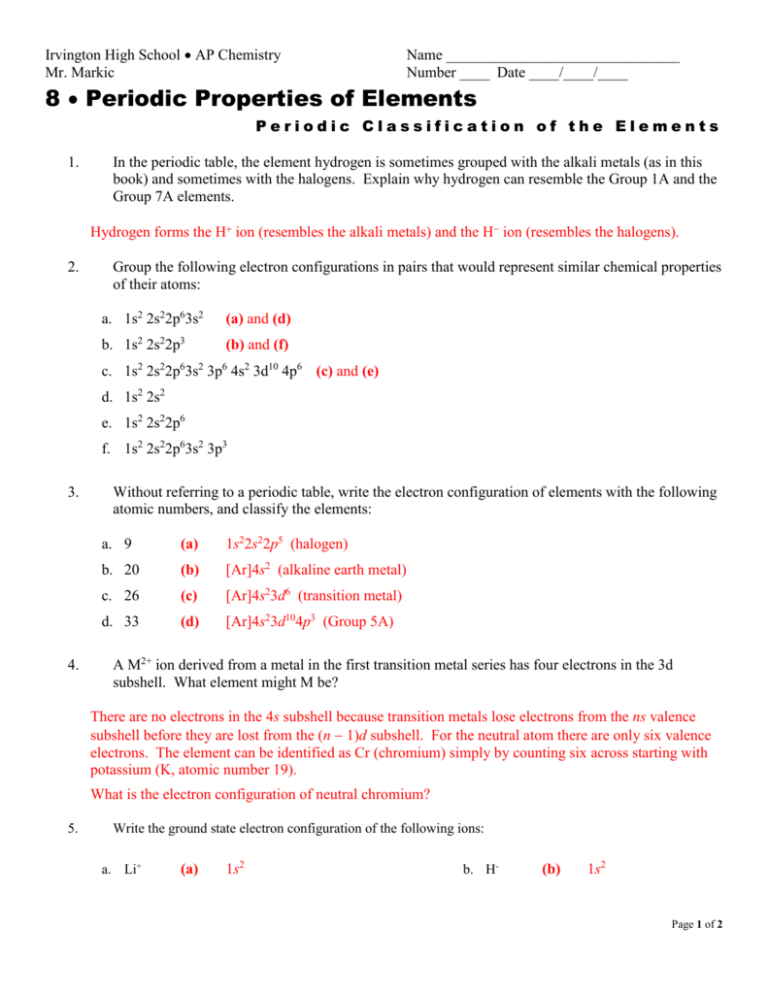
Irvington High School AP Chemistry Mr. Markic Name _______________________________ Number ____ Date ____/____/____ 8 Periodic Properties of Elements Periodic Classification of the Elements 1. In the periodic table, the element hydrogen is sometimes grouped with the alkali metals (as in this book) and sometimes with the halogens. Explain why hydrogen can resemble the Group 1A and the Group 7A elements. Hydrogen forms the H ion (resembles the alkali metals) and the H ion (resembles the halogens). 2. Group the following electron configurations in pairs that would represent similar chemical properties of their atoms: a. 1s2 2s22p63s2 (a) and (d) b. 1s2 2s22p3 (b) and (f) c. 1s2 2s22p63s2 3p6 4s2 3d10 4p6 (c) and (e) d. 1s2 2s2 e. 1s2 2s22p6 f. 1s2 2s22p63s2 3p3 3. 4. Without referring to a periodic table, write the electron configuration of elements with the following atomic numbers, and classify the elements: a. 9 (a) 1s22s22p5 (halogen) b. 20 (b) [Ar]4s2 (alkaline earth metal) c. 26 (c) [Ar]4s23d6 (transition metal) d. 33 (d) [Ar]4s23d104p3 (Group 5A) A M2+ ion derived from a metal in the first transition metal series has four electrons in the 3d subshell. What element might M be? There are no electrons in the 4s subshell because transition metals lose electrons from the ns valence subshell before they are lost from the (n 1)d subshell. For the neutral atom there are only six valence electrons. The element can be identified as Cr (chromium) simply by counting six across starting with potassium (K, atomic number 19). What is the electron configuration of neutral chromium? 5. Write the ground state electron configuration of the following ions: a. Li+ (a) 1s2 b. H- (b) 1s2 Page 1 of 2 c. N3- (c) 1s22s22p6 k. Sn2+ d. F- (d) 1s22s22p6 l. e. S2- (e) [Ne]3s23p6 Al3+ (f) g. Se2h. Br- f. 6. 7. (k) [Kr]5s24d10 (l) [Kr]5s24d105p6 m. Ba2+ (m) [Xe] [Ne] n. Pb2+ (n) [Xe]6s24f145d10 (g) [Ar]4s23d104p6 o. In3+ (o) [Kr]5d10 (h) [Ar]4s23d104p6 p. Tl+ (p) [Xe]6s24f145d10 q. Tl3+ (q) [Xe]4f145d10 i. Rb+ (i) [Kr] j. Sr2+ (j) [Kr] Te2- Write the ground state electron configuration of the following transition metal ions: a. Sc3+ (a) [Ar] i. Ni2+ (i) [Ar]3d8 b. Ti4+ (b) [Ar] j. Cu+ (j) [Ar]3d10 c. V5+ (c) [Ar] k. Cu2+ (k) [Ar]3d9 d. Cr3+ (d) [Ar]3d3 l. Ag+ (l) [Kr]4d10 e. Mn2+ (e) [Ar]3d5 m. Au+ (m) [Xe]4f145d10 f. Fe2+ (f) [Ar]3d6 n. Au3+ (n) [Xe]4f145d8 g. Fe3+ (g) [Ar]3d5 o. Pt2+ (o) [Xe]4f145d8 h. Co2+ (h) [Ar]3d7 Which of the following species are isoelectronic with each other? C, Cl-, Mn2+, B-, Ar, Zn, Fe3+, Ge2+ Two species are isoelectronic if they have the same number of electrons. Can two neutral atoms of different elements be isoelectronic? (a) C and B are isoelectronic. (b) Mn2 and Fe3 are isoelectronic. (c) Ar and Cl are isoelectronic. (d) Zn and Ge2 are isoelectronic. With which neutral atom are the positive ions in (b) isoelectronic? Page 2 of 2







