SCH 3U Gases Review 2004
advertisement

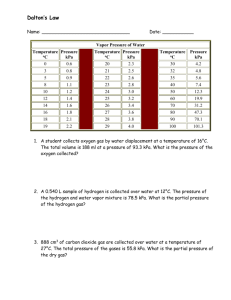
SCH 3U Multiple Choice 1. 2. Gas Review Answer Key What temperature on the Kelvin scale corresponds to 35oC? a) 135 K b) 238 K c) 293 K d) 308 K e) 333 K [solution: K = 35oC + 273 K = 308] A gas has a volume of 40 mL at 283 K and 94 kPa pressure. Which equation below will allow for the calculation of the volume of the gas in mL at 263 K and 103 kPa? (40 mL )(263 K)(94 kPa) (40 mL)(283 K)(94 kPa) a) b) (283 K)(103 kPa) (263 K)(103 kPa) c) (40 mL)(263 K)(103 kPa) (283 K)(94 kPa) d) (40 mL)(283 K)(103 kPa) (263 K)(94 kPa) (283 K) (94 kPa) e) (40 mL)(263 K)(103 kPa) p1v1 p2 v2 Solution: v2 p1v1 p2 so, v2 = (40 mL )(263 K)(94 kPa) (283 K)(103 kPa) 3. The molar volume of O2 gas refers to the volume occupied by a) 6.02 x 1023 molecules of O2 gas at STP or SATP b) 1 mole of O2 gas at STP or SATP c) 32.0 g of O2 gas at STP or SATP d) all of the above e) none of the above 4. Consider the balanced equation: 4NH3(g) + 3O2(g) The ratio of the reactants, (NH3 : O2) by volume is a) 4:3 b) 2:6 c) 7:8 2N2(g) + 6H2O(g) d) 3:2 e) 6:2 Solution: The Law of Combining Volumes tells us that the mole ratios in a balanced chemical equation can be used as volume ratios for gaseous reactants and products so the answer is a) 4:3. 5. Two diatomic gases, A2 and B2, combine chemically to form a compound. It is found that 2 moles of A2 require 3 moles of B2 to form 2 moles of the compound. Which of the following is the compound? a) A2B2 b) A2B3 c) A3B2 d) A3B e) AB Solution: 2A2(g) + 3B2(g) 2A2B3 6. Given the balanced chemical equation: 2SO2(g) + O2(g) 2SO3(g) o At 0 C and 90 kPa, 300 L of oxygen combine with sufficient sulfur dioxide to produce sulfur trioxide. What volume of sulfur trioxide will be produced? a) 300 L b) 450 L c) 600 L d) 750 L e) 800 L Solution: The Law of Combining Volumes tells us that the mole ratios in a balanced chemical equation can be used as volume ratios for gaseous reactants and products. So – if we have 300 L of oxygen gas reacting we’ll get 2 times as much SO3(g) or 600 L. Multiple Choice answers: 1d 2a 3d 4a 5b 6c Longer Problems PLEASE note that rearranging equations is YOUR JOB on an evaluation. The information provided here is to help you check over your problem solutions, only. 1. A certain volume of a gas has its temperature lowered from 50.0oC to 25.0oC. Pressure and amount of gas are held constant. If the final volume is 260. mL, what was the initial volume? (282 mL) Solution: t1 50. oC so T1 323 K V1 ? t2 25. C so T2 298 K V2 260 mL o Use Charles'Law. 260 mL 323 K v1 v2 T1 T2 298K v2T1 v1 T2 2. v1 the original volume was 282 mL. 282 mL v1 A gas occupies 450. mL at a temperature of 240. K and a pressure of 120. kPa. The conditions are changed so that the same amount of gas occupies a volume of 150. mL at a temperature of 300. K. What is the new pressure? (450 kPa) Solution: Use the combined gas law. (see next page…) v1 450. mL v2 150.mL T1 240.K T2 300.K p1 120.kPa p2 ? p1v1 p2 v2 T1 T2 p2 p1v1T2 Now, just substitute & solve v2T1 3. A volume of 8.00 L of O2 gas is warmed from 17.0oC to 307oC while the pressure and the amount of gas remain constant. What volume will the gas occupy at the higher temperature? (16.0 L) Solution Set Up: v1 v 2 T1 T2 v1 8.00 L v 2 ? rearranged as v 2 v1T2 T1 T1 290 K T2 580 K 4. 5. A gas occupies a volume of 18.0 L at a pressure of 88.7 kPa and a temperature of 127oC. What is its volume at STP? Solution Set Up: v1 18 .0 L v2 ? p1 88 .7 kPa p 2 101 kPa T1 400 K T2 273 K (10.8 L) v1 p1 v 2 p 2 v pT rearranged as v 2 1 1 2 T1 T2 T1 p 2 Do question 4 page 456. 1 2 2 2 2 1 2 [Answers: a) v x 3 x 1 = 3 of original v; (b) v x 1 x 1 = 4 times original v; (c) v x 2 x 1 = v same as original] 6. A gas bulb with a volume of 1.00 L contains a mass of 3.00 g of a compound in the gas phase at SATP. What is the molar mass of g the gas? (74.4 mol ) Solution Set Up: v 1.00 L p 100 kPa T 298 K m = 3.00 g Find n, using either pv = nRT (rearrange as n pv ) OR RT Use molar volume where at SATP we know that 1 mol of any gas occupies a volume of 24.8 L. so n v( Once you have found n, rearrange the equation n 7. 1 mol ) 24 .8 L m to solve for “mm”, molar mass. mm At 25.0oC and 60.0 kPa, 585 mL of an unknown gas has a mass of 2.42 g. a) Calculate the molar mass of the gas. g (171 mol ) Solution Set Up: See the set up for #6 above. This time, to calculate number of moles of gas, n, you will have to use pv = nRT (rearrange as shown above to solve for n) because you can only use molar volumes at STP or SATP. Once you have found n, rearrange the equation n m to solve for “mm”, molar mass. mm b) The unknown gas has the empirical formula CClF2.What is the molecular formula of the gas? (C2Cl2F4) Solution Set Up: Find the molar mass of the empircal formula, CClF2 . Because the molar mass is ½ that of your answer for part a) above, the molecular formula of the gas must be twice the empirical formula.. 8. Do #1 at the bottom of page 465 in Chemistry 11, Nelson. Your answer for part a) should be the equivalent of (385 – 240) kPa. 9. Assume 75.0 mL of carbon dioxide gas is collected over water at 23.0oC and 97.0 kPa. a) What is the pressure exerted by the dry carbon dioxide gas? (Use the water vapour pressure table pg 464 to help solve this problem!) (94.2 kPa) The pressure of the dry carbon dioxide gas will be the atmospheric pressure minus the pressure of the water vapour in the air at 23.0oC because the carbon dioxide was collected over water – so there is water vapour mixed in with it in its container. We know what to do here because Dalton’s Law of Partial Pressures states that ptotal = p for each gas present in a container. b) What volume would dry carbon dioxide gas occupy at SATP? (71.1 mL) p1 pressure of dry CO2 when collected ( see part a) p 2 100 kPa 94 .2 kPa 10. T1 296 K T2 298 K v1 0.075 L v2 ? Use the combined gas law. Rearrange as in #4. At STP, what volume of CO2 is required to produce 50.0 L of CO according to the balanced equation provided? CO2(g) + C(s) 2CO(g) (25.0 L) Solution: Use the Law of Combining Volumes. If you do not know it, it is on the question handout for this work and it is in the text, or you can ask your teacher to explain it again. 11. What mass of calcium carbide, CaC2(s) is needed to produce 235 mL of acetylene gas, C2H2(g), at STP from the balanced equation given below? (0.673 g) CaC2(s)+ H2O(l) C2H2(g) + Ca(OH)2(aq) Solution Set Up: Note: Pressure and temperature values will, generally, have an effect only on gases for the problems we solve. mCaC2(s) = ? VC2H2(g) = 0.235 L pC2H2(g) = 101 kPa TC2H2(g) = 273 K 12. So, use the ideal gas law to solve for n, the number of moles of C2H2(g) produced. Once you have that, you can use your knowledge of the mole ratios in balanced equations to say that number of moles of CaC2(s) needed will be equal to the number of moles of C2H2(g) produced. Once you have the number of moles of CaC2(s), you can solve for the mass . Aluminum metal is produced by the following process: 2 Al2O3(aq) 4 Al(s) + 3O2(g) What mass of aluminum metal is produced if 150. L of oxygen gas at 350.oC and 98.0 kPa is released? (102 g) Solution Set Up: Follow the same set up as for #11 above. It is the same problem with different given values and different mole ratios in the balanced chemical equation (i.e. This time the ratio of the number of moles of Al(s) produced to the number moles of oxygen gas is not 1:1 !!! 13. What volume of carbon dioxide gas measured at 65.0oC and 103.5 kPa will be produced from the decomposition of 20.85 g of baking soda, NaHCO3(s) as shown below? (3.37 L) 2 NaHCO3(s) Na2CO3(s) + CO2(g) + H2O(g) Solution Set Up: Note: The temperature and pressure information will not affect the solid. So, make 20.85 g of NaHCO3(s) into moles. Then use the molar ratios in the balanced chemical equation to calculate the number of moles of CO2(g) that will be produced using this amount of NaHCO3(s) . [This part is standard stuff you have been doing since unit 3…] Once you know the number of moles of carbon dioxide gas produced, you can use the ideal gas law, pv = nRT, to solve for its volume, v, in liters. 14. What mass of zinc must be reacted to form 850.0 mL of hydrogen gas at 95 kPa pressure and 17.0oC from the balanced equation below? (2.19 g) Zn(s) + HCl(aq) H2(g) + ZnCl2(aq) Solution Set Up: This time you need to find the number of moles of hydrogen gas produced first, using pv = nRT. Once you have that, you can use the molar ratios in the balanced equation to determine the number of moles of Zn(s) used. Then you convert the number of mole s of Zn(s) used to a mass value using n m . mm