Male reproductive system 1 & 2
advertisement

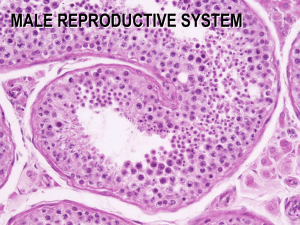
Anatomy and Human Biology 2214 August 18 and 24, 2009 M. Hall MALE REPRODUCTIVE SYSTEM Objectives: After you finish studying this lecture, you should be able to: Recognise and name the structures of the male reproductive system Understand the gross structure of the testis Identify all of the cells of the seminiferous epithelium Distinguish between spermatocytogenesis and spermiogenesis and describe the progression of sperm through the complete process of spermatogenesis Identify Sertoli cells and describe their main functions. Identify Leydig cells and describe their main functions Understand the hormonal control of spermatogenesis Identify the ducts involved in the passage of sperm from the testes to the penis, and describe what role each plays in the maturation of sperm Identify the accessory sex glands and know their role in nourishing and protecting the sperm after ejaculation Understand the structure of the penis and how blood flow causes erection Understand the mechanism of action of Viagra (if you understand this, then you will have answered the previous question). Understand the role of the sympathetic and parasympathetic nervous systems in erection and ejaculation. Distinguish between sperm and semen, and know the components of semen The male reproductive system consists of the testes, genital ducts, accessory glands and penis. (1) TESTES Embryology: The testes develop in the abdomen from the indifferent gonads of the embryo. Until the sixth week of development, the indifferent gonads, which consist of a cortex and a medulla, are identical in both sexes. In genetic males, the medulla develops 1 during the seventh and eighth weeks into a testis, and the cortex regresses. A specific gene on the Y-chromosome, the SRY gene is thought to produce a protein, the testes determining factor (TDF) which turns on the male genes responsible for producing the male hormones [androgens]. If this gene is not turned on, a female results. As fetal growth continues, the testes start to descend from their original position in the abdomen. They pass through the inguinal canal and reach the scrotum by birth. If the testes do not descend out of the body cavity, spermatogenesis is affected, and the male is sterile. (Temperature of testis is 2o lower than body temp). Functions: The testes have two interrelated functions: (1) the production of sperm (spermatogenesis) and (2) the production of steroid hormones (steroidogenesis). These hormones (primarily testosterone) function in the regulation of sperm development, and the growth, development and maintenance of the accessory reproductive glands. The steroid hormones also influence the development of secondary sex characteristics and to some extent, sexual behavior. Structure: Each testis is enclosed in a dense fibrous capsule, the tunica albuginea. The anterior and lateral surfaces of the testis are covered by a closed serous sac, the tunica vaginalis which is derived from the peritoneum. Along the posterior border of the testis, the tunica albuginea is thickened and projects into the interior, forming the mediastinum testis. Ducts, nerves and vessels exit and enter the testis through the mediastinum. Numerous thin fibrous septa extend from the tunica albuginea, dividing the testis into 200300 pyramidal lobules. Each lobule contains 1-4 highly coiled seminiferous tubules, immersed in a web of connective tissue rich in blood vessels, lymphatics, nerves and endocrine cells (Leydig cells). The seminiferous tubules are coiled loops that empty at both ends into a system of tubules within the mediastinum. From the mediastinum arise 12-20 tubules, the ductuli efferentes which join to the tubule system called the epididymis. Spermatozoa and testicular fluid are produced in the seminiferous tubules, and they pass through this system of tubules to reach the epididymis and thence onward to the urethra. Seminiferous tubules: The seminiferous tubules are long (30-80 cm), convoluted tubules, and are thus seen sectioned in various planes. They consist of (1) a thin tunic of fibrous connective tissue, (2) a basement membrane and (3) a complex stratified epithelium, the germinal epithelium. This epithelium consists of two types of cells: (1) the supporting cells called Sertoli cells and (2) the spermatogenic or reproductive cells in various stages of differentiation. The process by which the germ cells proliferate and transform into 2 spermatozoa is called spermatogenesis. Spermatogenesis can be divided into three phases: (1) Spermatocytogenesis - during which spermatogonia, (diploid, 2N) situated next to the basal lamina, divide mitotically, producing cells that eventually give rise to primary spermatocytes (diploid, 4N) (2) Meiosis - during which the 46 chromosomes (diploid, 4N) of a primary spermatocyte undergo two successive divisions, resulting in four cells called spermatids, each with only 23 chromosomes (haploid, N). (3) Spermiogenesis - during which the haploid spermatids go through a series of differentiations to produce haploid spermatozoa. All three phases of spermatogenesis take place within the seminiferous tubules, from the spermatogonia at the basement membrane to the mature spermatids at the lumen of the tubule. (Note: Although the spermatids are morphologically differentiated, they are not yet motile, a process which occurs in the epididymis). Identification of the various stages of development can be difficult, although if you remember that cells move from the basement membrane of the seminiferous tubule to the lumen with increasing differentiation, the task becomes easier. Spermatogonia are large, diploid germ cells, situated in the basal compartment of the seminiferous tubules. Subsequent to puberty, these cells are induced by testosterone to enter the cell cycle. Primary spermatocytes are found just above the spermatogonia and are quite numerous. They are large cells with a more darkly staining nucleus, often seen with dividing chromosomes. As they migrate between the Sertoli cells, they form zonula occludens with these cells, thus maintaining the integrity of the blood-testis barrier. Secondary spermatocytes are not often seen as they divide soon after formation, each giving rise to two spermatids. Spermatids are seen in two stages--early and late stage. Early spermatids are round and cellular. They are smaller than spermatocytes with small nuclei containing condensed chromatin, and they usually lie close to the lumen. Late spermatids are easily recognized as they have begun to take the shape of spermatozoa. They are closely associated with the Sertoli cells, often with the residual body still attached. The process of spermiogenesis, or maturation of the spermatids to spermatozoa, 3 occurs while the spermatids are attached to the Sertoli cells. Mature spermatozoa are released into the lumen of the seminiferous tubule and undergo further maturation to become motile and hence fertile, as they pass through the epididymis. In humans, it takes about 64 days for a spermatogonium to complete the process of spermatogenesis, (spermatogonium to spermatozoan) and about 12 days for the spermatozoa to pass through the epididymis. Spermatogenesis occurs in a wavelike fashion. Thus different regions of the same seminiferous tubule exhibit different phases of spermatogenesis. You may see tubules that contain late spermatids, while other regions contain spermatozoa. Thus the three phases of spermatogenesis can be represented as follows: spermatocytogenesis meiosis _______ spermatogonia (2N)-primary spermatocytes(4N)-secondary spermatocytes(2N)-spermatids(N) spermiogenesis spermatids(N)--spermatozoa(N). Sertoli cells: The Sertoli cells are the supportive cells of the seminiferous tubules. They are tall columnar cells extending from the basement membrane to the lumen of the tubule. Each Sertoli cell surrounds its neighboring spermatogenic cells and fills up all the spaces between them. Specialized tight junctions (zonula occludens) are formed between adjacent Sertoli cells, thus producing a blood-testis barrier, which maintains a microenvironment for the spermatogenic cells to proceed through meiosis and differentiation to spermatozoa. The young spermatocytes must pass through the junctional complexes in order to reach the lumen. Sertoli cells have at least four main functions in the development and differentiation of the spermatogenic cells. 1. Support, protection and nutritional regulation of the developing spermatozoa. During spermatogenesis, the cells are constantly surrounded and supported by the Sertoli cells. The presence of tight junctions at the base of these cells isolates the developing spermatids from the blood supply and protects them from immunologic attack. These junctions also ensure that the spermatogenic cells depend on the Sertoli cells to mediate the exchange of nutrients. 2. Phagocytosis. The Sertoli cells phagocytize the residual bodies released from the spermatids during spermiogenesis. 3. Secretion. Sertoli cells continuously secrete a fluid (testicular fluid) into the lumen of the seminiferous tubule that nourishes and facilitates the transport of sperm to the genital ducts. 4. Production of Androgen-binding protein (ABP). which binds testosterone, thus concentrating this hormone in the seminiferous epithelium where it is essential for spermatogenesis. They also secrete a peptide called inhibin, which suppresses FSH synthesis by the anterior pituitary. 4 Sertoli cells Leydig cells Leydig (interstitial) cells: Leydig cells are large and ovoid in shape and are situated in the connective tissue between the tubules. They are endocrine cells and thus are associated with blood vessels. These cells produce the male hormone testosterone, an adequate supply of which is necessary for the growth and secretory activity of the accessory sex glands, i.e. seminal vesicles, prostate and bulbourethral glands. A high local level of testosterone within the seminiferous tubules is also necessary for sperm production. Testosterone is also responsible for the development and maintenance of secondary sex characteristics, such as facial and pubic hair, low voice, muscularity, behavior, libido, etc. Testosterone levels are quite low from before birth until puberty, when they increase in response to the stimulus of luteinizing hormone (LH) from the pituitary. (Coincidentally, this increase in testosterone levels appears to be a signal for the migration of the male brain down to the penis, where it resides for the next 40 or 50 years). Hormonal control of spermatogenesis: Both the endocrine function (production of testosterone) and the exocrine function (spermatogenesis) of the testes are strongly regulated by the pituitary gonadotrophic hormones, luteinizing hormone (LH) and follicle stimulating hormone (FSH). The endocrine function of the testis primarily resides in the Leydig cells, which synthesize and secrete the principal androgen, testosterone. As the testosterone leaves the Leydig cells, it may pass into the blood, or it may pass through the peritubular tissue, and enter the seminiferous tubules. High local levels of testosterone within the testis (greater than 200 times the circulating levels) are necessary for the proliferation and differentiation of the spermatogenic cells within the seminiferous epithelium. The lower circulating level of testosterone influences (1) the growth and maintenance of secondary sexual characteristics (such as growth of beard, distribution of hair and low-pitched voice): (2) the growth and maintenance of the accessory sex glands (seminal vesicles, prostate and bulbourethral), genital duct system and external genitalia: (3) anabolic and general metabolic processes (such as skeletal growth, muscle growth, fat distribution) and (4) behavior, including libido, channel surfing and watching the West Coast Eagles. 5 The anterior pituitary produces two principal hormones involved in this process: luteinizing hormone (LH) and follicle cell stimulating hormone (FSH). LH is released by the pituitary in response to low serum testosterone levels and stimulates Leydig cells to produce testosterone from cholesterol. Because blood testosterone levels are not sufficient to initiate and maintain spermatogenesis, FSH, also from the anterior pituitary, stimulates the Sertoli cells to synthesize and release Androgen binding protein (ABP). The function of this protein is to bind testosterone, thereby establishing a high local concentration (200x) of this hormone in the seminiferous tubules and the initial portion of the duct system. This elevated level is required to sustain spermatogenesis. A second important substance produced by the Sertoli is Inhibin, which functions in the regulation of the FSH levels and thus aids in regulating the number of cells entering spermatogenesis. 2). GENITAL DUCT SYSTEM After production in the seminiferous tubules, the spermatozoa are transported through a series of ducts and stored until they are expelled through the penis during ejaculation. Feeding into this extended tubular system are the accessory sex glands: the seminal vesicles, prostate gland and bulbourethral gland. The seminiferous tubules connect with a series of channels within the dense connective tissue of the mediastinum, called the rete testis. At the superior end of the 6 mediastinum, 12 to 20 efferent ducts leave the testis and coil into a mass to form the head of the epididymis. These efferent ducts are lined with a pseudostratified columnar epithelium, consisting of alternating groups of tall columnar and cuboidal cells, both of which may be ciliated or non-ciliated. The differing cell height gives this tubule a wavy appearance. The ciliated cells aid in the transport of sperm and fluids, while the nonciliated cells possess numerous microvilli and have an absorptive function (most of the fluids produced within the seminiferous tubules are absorbed in the efferent ducts and the epididymis). The efferent ducts join to form a single highly convoluted ductus epididymis, which is about 6 meters long. It consists of a head, a body and a tail. The ductus epididymis is lined with a pseudostratified columnar epithelium consisting of two main cell types -small basal cells lying near the basement membrane, and tall columnar cells (principal cells) whose surfaces are covered with non-motile stereocilia (These are not cilia at all but long microvilli). Most of the fluid that is not absorbed by the efferent ducts is absorbed in the epididymis. The tubule of the epididymis is surrounded by a thin coat of smooth muscle whose peristaltic contractions help to move sperm along the duct. The tail of the epididymis is the principal site of sperm storage. The epydidymus also provides the essential environment and some of the molecular products required for maturation of the sperm. This maturation, like their earlier differentiation, is androgen dependent. It is within the epididymis that spermatozoa become motile and hence fertile. The head and most of the body of the epididymis contain only a thin layer of smooth muscle, which undergoes rhythmic peristaltic contractions to move the sperm along the duct. The tail contains three distinct muscle layers, similar to that seen in the vas deferens. Intense contractions of these three muscle layers, after appropriate neural stimulation, force the sperm into the vas deferens during ejaculation. The vas deferens begins as a direct continuation of the ductus epididymis and eventually empties into the prostatic urethra. The epithelium of the vas deferens is pseudostratified and stereociliated along most of its length. The wall of the vas is composed of three layers of smooth muscle, an internal longitudinal, a middle circular, and an outer longitudinal layer, as seen in the tail of the epididymis. An extensive myenteric nerve plexus is present in these muscle layers. Sympathetic nervous stimulation of the smooth muscle surrounding the vas deferens causes powerful contractions that expel the spermatozoa at ejaculation. The adventitia of the vas deferens blends with the tissues of the spermatic cord through which the vascular and neural supply to the testes are carried. Vasectomy (male sterilization) is simply performed by cutting both of the vas deferens, and tying the cut ends. This very effectively prevents sperm from being added to all of the other components of semen during ejaculation. 7 3). ACCESSORY SEX GLANDS Feeding into the duct system which conducts sperm from the testes to the urethra are three accessory sex glands -- the seminal vesicles, prostate and bulbourethral glands. Each seminal vesicle is a long, convoluted tubular structure which opens into the vas deferens just as it enters the prostate gland. (As it is a highly convoluted tube, it is seen in different orientations on sectioning). The pseudostratified epithelium consists of tall or short columnar cells and basal cells ,arranged irregularly along the basement membrane. These cells produce a thick secretion, rich in fructose, flavins, vitamin C and prostaglandins. This secretion, which is stored in the seminal vesicles, is eliminated during ejaculation by the contraction of its smooth muscle coat, and provides nutrient material for the spermatozoa. The activity of the gland is controlled by the level of testosterone in the blood and thus varies with the age of the individual. Cells of the seminal vesicle Structure of Prostate gland 8 The prostate gland is a collection of 30-50 branched tubuloalveolar glands whose ducts converge into about 20 terminal ducts that open into that part of the urethra called the prostatic urethra. The prostate produces prostatic fluid and stores it in its interior for expulsion during ejaculation. This fluid is rich in citric acid, acid phosphatase, fibrinolysin and proteolytic enzymes that function in liquefaction of the semen after ejaculation. This allows the sperm to start their journey to the Fallopian tubule to fertilize the egg. The secretion of the gland is controlled by the level of testosterone. The gland is surrounded by a fibroelastic capsule, rich in smooth muscle. Contraction of this smooth muscle, as well as of smooth muscle in the mucosa of the glandular epithelium, expels the prostatic fluid during ejaculation. The cells of the prostatic epithelium vary from simple cuboidal or columnar to pseudostratified columnar. A characteristic feature of the prostate is the presence of prostatic concretions (corpora amylacea) which are glycoprotein-rich bodies that are often calcified. Their number increases with age. As men age, the stroma and parenchyma begin to hypertrophy, a condition known as benign prostatic hypertrophy. The enlarged prostate partly constricts the urethra, resulting in difficulties with urination. Adenocarcinoma of the prostate is a common form of cancer in men, which affects 30% of the male population over the age of 75. It is a slow growing but highly metastatic form of cancer. However, a simple blood test can detect the prostate specific antigen (PSA) which allows early detection of this condition. The paired bulbourethral glands open into the urethra at the base of the penis. During arousal, they produce a clear, alkaline, viscous secretion that coats the lining of the urethra and acts as a lubricant for the spermatozoa as they travel through the urethra. This secretion also neutralizes residual urine in the urethra, the acidity of which would be harmful to the spermatozoa. 4). PENIS The penis is a structure which, when erect, can conduct sperm into the vagina. This organ consists of three cylindrical masses of erectile tissue - two corpora cavernosa which lie above a single corpus spongiosum, The urethra passes through the corpus spongiosum. A dense fibrous coat, the tunica albuginea surrounds each of the three bodies, and all three of these erectile bodies are surrounded by a layer of dense connective tissue and covered with thin skin and a stratified squamous epithelium. The erectile structures consist of numerous interconnected cavernous spaces created by a network of fine trabeculae that contain fibroelastic tissue and smooth muscle. The spaces are lined with typical vascular endothelium. The penis becomes erect when the trabecular spaces are distended with blood. Blood enters the cavernous spaces from the helicine arteries. Real or imaginary erotic stimuli evoke parasympathetic activity that dilates the helicine arteries resulting in an increased blood flow through them, thus causing the cavernous sinuses to fill with blood and the penis to become erect. Back-flow through peripheral veins is obstructed by compression against the tunica albuginea, further contributing to maintenance of the erection. Sympathetic stimulation of the smooth muscle of the arteries at the end of ejaculation decreases arterial blood flow and increases venous drainage, resulting in detumescence and return to the flaccid state. 9 Structure of penis Blood flow to erectile tissue Viagra: The physiologic mechanism of erection of the penis involves the production of nitric oxide (NO) by NO synthase during sexual stimulation. Parasympathetic impulses triggered during sexual arousal cause the local release of NO, which activates the enzyme guanylate cyclase in the smooth muscle cells surrounding the arteries leading to the trabecular meshwork and increasing the levels of cGMP. Increased cGMP and decreased calcium causes relaxation of the smooth muscle of the arteries feeding into the penis and probably of smooth muscle in the trabeculae of the corpus cavernosum, increasing the flow of blood into the penis, resulting in erection. After orgasm, the enzyme phosphodiesterase type 5, (PDE 5) causes the hydrolysis of cGMP, constriction of the arteries and decrease of blood flow into the erectile tissue, and thus detumescence. Viagra acts by inhibiting PDE 5. When sexual stimulation causes local release of NO and the production of cGMP, inhibition of -PDE 5 by Viagra causes increased and sustained levels of cGMP, resulting in continued smooth muscle relaxation and inflow of blood to the corpus cavernosum. This can result in the maintenance of erection for 2-4 hours. Viagra is highly selective for PDE 5, which is important since a very similar enzyme, PDE 3, is involved in cardiac contractility. The only known -PDE that is also affected by Viagra is PDE 6, which is present in the retina, probably resulting in the increased blue color sensitivity reported by a small percentage of Viagra users. (I bet you never thought that sex could be this dull!!). 10 Mechanism of action of Viagra Semen: In addition to spermatozoa, the semen contains contributions from the accessory sex glands. Ejaculation is initiated by sympathetic stimulation of the smooth muscle bundles associated with the genital ducts and accessory sex glands. Contraction of these muscles forces the secretory products from the glands and propels the sperm and fluids along the duct system. At ejaculation, the different components of the semen usually follow one another in a definite sequence. 1. During arousal, mucous-like fluids are released from the bulbourethral glands and act as a lubricant. 2. As ejaculation begins, the acid phosphatase and citric acid-rich secretions of the prostate gland are released. 3. The spermatozoa then follow these secretions. 4. The seminal vesicles then add the final component, a substance rich in fructose, which nourishes the sperm while in the vagina. The volume of the ejaculate in humans is 2-6 ml, 95% of which is contributed by the accessory glands. The average sperm density is about 100 million/ml -- compare this with one egg released each month in the female. Only a few hundred sperm reach the ampulla region of the oviduct, where fertilization occurs. A little known fact... The first testicular guard (Box) was used in cricket in 1874 and the first helmet was used in 1974. It took 100 years for men to realize that the brain is also important! 11