Gen Chemistry 2 1. Define the First Law of Thermodynamics The
advertisement

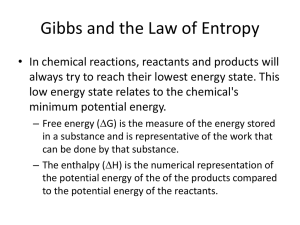
Gen Chemistry 2 1. Define the First Law of Thermodynamics The First Law of Thermodynamics is also known as the Law of Conservation of Energy. It states that the total energy of an isolated system remains constant and can only be transferred from one form to another. Energy can neither be created nor destroyed. 2. Define the following: a. E + Positive Internal Energy. Increase in temp of system energy. Decrease in temp of system. c. +q Energy given to the system e. +w Work done on system b. E – Negative internal d. –q Energy expended by the system f. –w Work done by system g. P V work PV Work happens when volume of a system changes h. state function A function that is characterized by its present states and not by the path taken to arrive at that state. i. H Sum of internal energy and product of volume and pressure. H = U + PV. 3. When is H equal to E? How should the relationship be expressed when there is a change in the number of moles of gas in a reaction? H is equal to E when number of moles in a system remain constant. H = E + d(PV) = E + d(nRT) = E + R d(nT) , d represents a change 4. What is the difference between a spontaneous and a non-spontaneous change? A spontaneous change is the one that occurs by itself and does not require any energy input. Free energy is released and the system moves to a lower energy thermodynamically stable state. Whereas non spontaneous change requires energy to take place. Change in gibbs free energy is negative for spontaneous change. 5. Why are reactions in which H = - often spontaneous? Negative enthalpy implies a release of energy by the system. This leads to the system arriving a lower energy thermodynamically stable state. This is the reason why reactions with negative H are often spontaneous. 6. Define the term ”entropy” and state the symbol used for this term. How is the magnitude of this value related to spontaneity of a reaction? Entropy is a state function often taken as a measure of degree of randomness in a system. Higher the entropy higher is the instability of the system. For a reversible process it is defined as the heat absorbed or emitted divided by its thermodynamic temperature. It is denoted by the symbol S. If entropy is positive the reaction is spontaneous and if it is negative the reaction is non spontaneous. 7. Explain how each of the following affects the entropy of a system: • Volume - Greater the volume greater the entropy. As change is entropy is given by the formula = nR ln(V2/V1) • Temperature – As temperature increases, entropy increases as energy of particles increases and hence the randomness • Physical state – Solids have lesser entropy whereas gases and liquids have higher entropies. • Number of particles – More the no of particles more the randomness and hence more the entropy. 8. Which three factors control spontaneity of a reaction? Temperature, Enthalpy and Entropy (as three affect gibbs free energy) 9. What happens in a reaction if T S (total) is greater than 0 and H system – T S (system) is less than 0 ? The change in gibbs free energy is negative and hence the reaction is spontaneous. Entropy change is also positive and hence the reaction will be spontaneous. 10. Define the term “Gibbs Free Energy” Summarize the relationship between G, H and S (on the back of this paper) 11. What does it mean when G = 0? __________________ When G is positive___________________________________ When G is negative __________________________________ 12.What is the difference between G0 and G?